CD40 regulates the processing of NF-kappaB2 p100 to p52
- PMID: 12374738
- PMCID: PMC129074
- DOI: 10.1093/emboj/cdf542
CD40 regulates the processing of NF-kappaB2 p100 to p52
Abstract
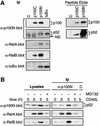
The nf-kb2 gene encodes the cytoplasmic NF-kappaB inhibitory protein p100 from which the active p52 NF-kappaB subunit is derived by proteasome-mediated proteolysis. Ligands which stimulate p100 processing to p52 have not been defined. Here, ligation of CD40 on transfected 293 cells is shown to trigger p52 production by stimulating p100 ubiquitylation and subsequent proteasome-mediated proteolysis. CD40-mediated p52 accumulation is dependent on de novo protein synthesis and triggers p52 translocation into the nucleus to generate active NF-kappaB dimers. Endogenous CD40 ligation on primary murine splenic B cells also stimulates p100 processing, which results in the delayed nuclear translocation of p52-RelB dimers. In both 293 cells and primary splenic B cells, the ability of CD40 to trigger p100 processing requires functional NF-kappaB-inducing kinase (NIK). In contrast, NIK activity is not required for CD40 to stimulate the degradation of IkappaBalpha in either cell type. The regulation of p100 processing by CD40 is likely to be important for the transcriptional regulation of CD40 target genes in adaptive immune responses.
Figures







References
-
- Aizawa S. et al. (1997) Tumor necrosis factor receptor-associated factor (TRAF) 5 and TRAF2 are involved in CD30-mediated NF-κB activation. J. Biol. Chem., 272, 2042–2045. - PubMed
-
- Baeuerle P.A. and Henkel,T. (1994) Function and activation of NF-κB in the immune system. Annu. Rev. Immunol., 12, 141–179. - PubMed
-
- Beinke S., Belich,M.P. and Ley,S.C. (2002) The death domain of NF-κB1 p105 is essential for signal-induced p105 proteolysis. J. Biol. Chem., 277, 24162–24168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

