A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation
- PMID: 12374740
- PMCID: PMC129083
- DOI: 10.1093/emboj/cdf551
A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation
Abstract
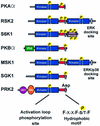
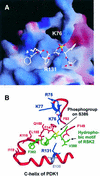
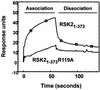
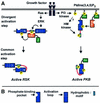
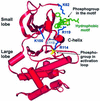
The growth factor-activated AGC protein kinases RSK, S6K, PKB, MSK and SGK are activated by serine/threonine phosphorylation in the activation loop and in the hydrophobic motif, C-terminal to the kinase domain. In some of these kinases, phosphorylation of the hydrophobic motif creates a specific docking site that recruits and activates PDK1, which then phosphorylates the activation loop. Here, we discover a pocket in the kinase domain of PDK1 that recognizes the phosphoserine/phosphothreonine in the hydrophobic motif by identifying two oppositely positioned arginine and lysine residues that bind the phosphate. Moreover, we demonstrate that RSK2, S6K1, PKBalpha, MSK1 and SGK1 contain a similar phosphate-binding pocket, which they use for intramolecular interaction with their own phosphorylated hydrophobic motif. Molecular modelling and experimental data provide evidence for a common activation mechanism in which the phosphorylated hydrophobic motif and activation loop act on the alphaC-helix of the kinase structure to induce synergistic stimulation of catalytic activity. Sequence conservation suggests that this mechanism is a key feature in activation of >40 human AGC kinases.
Figures









References
-
- Alessi D.R., James,S.R., Downes,C.P., Holmes,A.B., Gaffney,P.R., Reese,C.B. and Cohen,P. (1997) Characterization of a 3-phospho inositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol., 7, 261–269. - PubMed
-
- Alessi D.R., Kozlowski,M.T., Weng,Q.P., Morrice,N. and Avruch,J. (1998) 3-phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. - PubMed
-
- Balendran A., Biondi,R.M., Cheung,P.C., Casamayor,A., Deak,M. and Alessi,D.R. (2000) A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Cζ (PKCζ) and PKC-related kinase 2 by PDK1. J. Biol. Chem., 275, 20806–20813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

