Protection of the intestinal mucosa by intraepithelial gamma delta T cells
- PMID: 12376619
- PMCID: PMC137885
- DOI: 10.1073/pnas.212290499
Protection of the intestinal mucosa by intraepithelial gamma delta T cells
Abstract
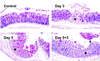
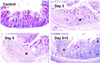
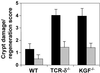
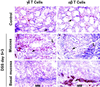
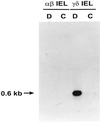
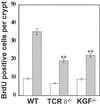
gammadelta intraepithelial T lymphocytes (IEL) represent a major T cell population within the intestine of unclear functional relevance. The role of intestinal gammadelta IEL was evaluated in the dextran sodium sulfate (DSS) induced mouse colitis model system. Large numbers of gammadelta T cells, but not alphabeta T cells, were localized at sites of DSS-induced epithelial cell damage. gammadelta IEL in DSS treated mice expressed keratinocyte growth factor (KGF), a potent intestinal epithelial cell mitogen. gammadelta cell-deficient mice (TCRdelta(-/-)) and KGF-deficient mice (KGF(-/-)), but not alphabeta cell-deficient mice (TCRalpha(-/-)), were more prone than wild-type mice to DSS-induced mucosal injury and demonstrated delayed tissue repair after termination of DSS treatment. Termination of DSS treatment resulted in vigorous epithelial cell proliferation in wild-type mice but not in TCRdelta(-/-) mice or KGF(-/-) mice. These results suggest that gammadelta IEL help preserve the integrity of damaged epithelial surfaces by providing the localized delivery of an epithelial cell growth factor.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

