The calcium-binding activity of a vacuole-associated, dehydrin-like protein is regulated by phosphorylation
- PMID: 12376635
- PMCID: PMC166597
- DOI: 10.1104/pp.002550
The calcium-binding activity of a vacuole-associated, dehydrin-like protein is regulated by phosphorylation
Abstract
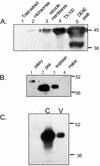
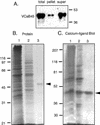
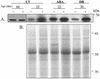
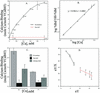
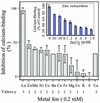
A vacuole membrane-associated calcium-binding protein with an apparent mass of 45 kD was purified from celery (Apium graveolens). This protein, VCaB45, is enriched in highly vacuolate tissues and is located within the lumen of vacuoles. Antigenically related proteins are present in many dicotyledonous plants. VCaB45 contains significant amino acid identity with the dehydrin family signature motif, is antigenically related to dehydrins, and has a variety of biochemical properties similar to dehydrins. VCaB45 migrates anomalously in sodium dodecyl sulfate-polyacrylamide gel electrophoresis having an apparent molecular mass of 45 kD. The true mass as determined by matrix-assisted laser-desorption ionization time of flight was 16.45 kD. VCaB45 has two characteristic dissociation constants for calcium of 0.22 +/- 0.142 mM and 0.64 +/- 0.08 mM, and has an estimated 24.7 +/- 11.7 calcium-binding sites per protein. The calcium-binding properties of VCaB45 are modulated by phosphorylation; the phosphorylated protein binds up to 100-fold more calcium than the dephosphorylated protein. VCaB45 is an "in vitro" substrate of casein kinase II (a ubiquitous eukaryotic kinase), the phosphorylation resulting in a partial activation of calcium-binding activity. The vacuole localization, calcium binding, and phosphorylation of VCaB45 suggest potential functions.
Figures





References
-
- Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular calcium waves. Cell. 1995;82:765–771. - PubMed
-
- Campbell KP, MacLennan DH, Jorgensen AO. Staining of calcium-binding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye, “stains-all.”. J Biol Chem. 1983a;258:11267–11273. - PubMed
-
- Campbell KP, MacLennan DH, Jorgensen AO. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem. 1983b;258:1197–1204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

