Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis
- PMID: 12376641
- PMCID: PMC166603
- DOI: 10.1104/pp.008110
Molecular characterization of a heteromeric ATP-citrate lyase that generates cytosolic acetyl-coenzyme A in Arabidopsis
Abstract
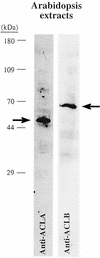
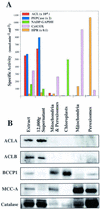
Acetyl-coenzyme A (CoA) is used in the cytosol of plant cells for the synthesis of a diverse set of phytochemicals including waxes, isoprenoids, stilbenes, and flavonoids. The source of cytosolic acetyl-CoA is unclear. We identified two Arabidopsis cDNAs that encode proteins similar to the amino and carboxy portions of human ATP-citrate lyase (ACL). Coexpression of these cDNAs in yeast (Saccharomyces cerevisiae) confers ACL activity, indicating that both the Arabidopsis genes are required for ACL activity. Arabidopsis ACL is a heteromeric enzyme composed of two distinct subunits, ACLA (45 kD) and ACLB (65 kD). The holoprotein has a molecular mass of 500 kD, which corresponds to a heterooctomer with an A(4)B(4) configuration. ACL activity and the ACLA and ACLB polypeptides are located in the cytosol, consistent with the lack of targeting peptides in the ACLA and ACLB sequences. In the Arabidopsis genome, three genes encode for the ACLA subunit (ACLA-1, At1g10670; ACLA-2, At1g60810; and ACLA-3, At1g09430), and two genes encode the ACLB subunit (ACLB-1, At3g06650 and ACLB-2, At5g49460). The ACLA and ACLB mRNAs accumulate in coordinated spatial and temporal patterns during plant development. This complex accumulation pattern is consistent with the predicted physiological needs for cytosolic acetyl-CoA, and is closely coordinated with the accumulation pattern of cytosolic acetyl-CoA carboxylase, an enzyme using cytosolic acetyl-CoA as a substrate. Taken together, these results indicate that ACL, encoded by the ACLA and ACLB genes of Arabidopsis, generates cytosolic acetyl-CoA. The heteromeric organization of this enzyme is common to green plants (including Chlorophyceae, Marchantimorpha, Bryopsida, Pinaceae, monocotyledons, and eudicots), species of fungi, Glaucophytes, Chlamydomonas, and prokaryotes. In contrast, all known animal ACL enzymes have a homomeric structure, indicating that a evolutionary fusion of the ACLA and ACLB genes probably occurred early in the evolutionary history of this kingdom.
Figures







Similar articles
-
Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis.Plant Cell. 2005 Jan;17(1):182-203. doi: 10.1105/tpc.104.026211. Epub 2004 Dec 17. Plant Cell. 2005. PMID: 15608338 Free PMC article.
-
Molecular biology of cytosolic acetyl-CoA generation.Biochem Soc Trans. 2000 Dec;28(6):593-5. Biochem Soc Trans. 2000. PMID: 11171137
-
ATP-citrate lyase is required for production of cytosolic acetyl coenzyme A and development in Aspergillus nidulans.Eukaryot Cell. 2010 Jul;9(7):1039-48. doi: 10.1128/EC.00080-10. Epub 2010 May 21. Eukaryot Cell. 2010. PMID: 20495057 Free PMC article.
-
Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: Pathway stoichiometry, free-energy conservation and redox-cofactor balancing.Metab Eng. 2016 Jul;36:99-115. doi: 10.1016/j.ymben.2016.03.006. Epub 2016 Mar 23. Metab Eng. 2016. PMID: 27016336 Review.
-
ATP-citrate lyase: a mini-review.Biochem Biophys Res Commun. 2012 May 25;422(1):1-4. doi: 10.1016/j.bbrc.2012.04.144. Epub 2012 May 3. Biochem Biophys Res Commun. 2012. PMID: 22575446 Review.
Cited by
-
Evidence for autotrophic CO2 fixation via the reductive tricarboxylic acid cycle by members of the epsilon subdivision of proteobacteria.J Bacteriol. 2005 May;187(9):3020-7. doi: 10.1128/JB.187.9.3020-3027.2005. J Bacteriol. 2005. PMID: 15838028 Free PMC article.
-
An Arabidopsis mutant line lacking the mitochondrial calcium transport regulator MICU shows an altered metabolite profile.Plant Signal Behav. 2023 Dec 31;18(1):2271799. doi: 10.1080/15592324.2023.2271799. Epub 2023 Oct 25. Plant Signal Behav. 2023. PMID: 37879964 Free PMC article.
-
Isoprenoid biosynthesis in dandelion latex is enhanced by the overexpression of three key enzymes involved in the mevalonate pathway.BMC Plant Biol. 2017 May 22;17(1):88. doi: 10.1186/s12870-017-1036-0. BMC Plant Biol. 2017. PMID: 28532507 Free PMC article.
-
Reverse genetic characterization of cytosolic acetyl-CoA generation by ATP-citrate lyase in Arabidopsis.Plant Cell. 2005 Jan;17(1):182-203. doi: 10.1105/tpc.104.026211. Epub 2004 Dec 17. Plant Cell. 2005. PMID: 15608338 Free PMC article.
-
Transcriptomic analysis revealed the mechanism of oil dynamic accumulation during developing Siberian apricot (Prunus sibirica L.) seed kernels for the development of woody biodiesel.Biotechnol Biofuels. 2015 Feb 22;8:29. doi: 10.1186/s13068-015-0213-3. eCollection 2015. Biotechnol Biofuels. 2015. PMID: 25834637 Free PMC article.
References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson M, Roberts JA. Arabidopsis: Annual Plant Reviews. Vol. 1. Boca Raton, FL: CRC Press; 1998.
-
- Arnold GW, Hill JJ. Chemical factors affecting the selection of food plants by ruminants. In: Harborne J, editor. Phytochemical Ecology. New York: Academic Press; 1972. pp. 71–101.
-
- Back K, He S, Kim KU, Shin DH. Cloning and bacterial expression of sesquiterpene cyclase, a key branch point enzyme for the synthesis of sesquiterpenoid phytoalexin capsidiol in UV-challenged leaves of Capsicum annuum. Plant Cell Physiol. 1998;39:899–904. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

