Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single-cell, facultative C(4) system of Hydrilla verticillata
- PMID: 12376652
- PMCID: PMC166614
- DOI: 10.1104/pp.008045
Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single-cell, facultative C(4) system of Hydrilla verticillata
Abstract
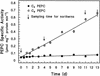
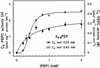
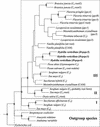
The submersed monocot Hydrilla verticillata (L.f.) Royle is a facultative C(4) plant. It typically exhibits C(3) photosynthetic characteristics, but exposure to low [CO(2)] induces a C(4) system in which the C(4) and Calvin cycles co-exist in the same cell and the initial fixation in the light is catalyzed by phosphoenolpyruvate carboxylase (PEPC). Three full-length cDNAs encoding PEPC were isolated from H. verticillata, two from leaves and one from root. The sequences were 95% to 99% identical and shared a 75% to 85% similarity with other plant PEPCs. Transcript studies revealed that one isoform, Hvpepc4, was exclusively expressed in leaves during C(4) induction. This and enzyme kinetic data were consistent with it being the C(4) photosynthesis isoform. However, the C(4) signature serine of terrestrial plant C(4) isoforms was absent in this and the other H. verticillata sequences. Instead, alanine, typical of C(3) sequences, was present. Western analyses of C(3) and C(4) leaf extracts after anion-exchange chromatography showed similar dominant PEPC-specific bands at 110 kD. In phylogenetic analyses, the sequences grouped with C(3), non-graminaceous C(4), and Crassulacean acid metabolism PEPCs but not with the graminaceous C(4), and formed a clade with a gymnosperm, which is consistent with H. verticillata PEPC predating that of other C(4) angiosperms.
Figures

 , The Ser residue that is common to all plant
PEPCs and that is the target for phosphorylation; ●, the unique
Hvpepc4 Met residue;
, The Ser residue that is common to all plant
PEPCs and that is the target for phosphorylation; ●, the unique
Hvpepc4 Met residue;  , the F. trinervia
putative C4 Lys site; ○, the unique
Hvpepc5 Val; and ★, the position of the
C4 signature Ser.
, the F. trinervia
putative C4 Lys site; ○, the unique
Hvpepc5 Val; and ★, the position of the
C4 signature Ser.

References
-
- Björkman O, Pearcy RW, Nobs MA. Hybrids between Atriplexspecies with and without β-carboxylation photosynthesis: photosynthetic characteristics. Carnegie Inst Wash Year Book. 1970;69:640–648.
-
- Bläsing OE, Westhoff P, Svensson P. Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria, a conserved serine residue in the carboxyl-terminal part of the enzyme is a major determinant for C4-specific characteristics. J Biol Chem. 2000;275:27917–27923. - PubMed
-
- Bowes G, Rao SK, Estavillo GM, Reiskind JB. C4 mechanisms in aquatic angiosperms: comparisons with terrestrial C4systems. Funct Plant Biol. 2002;29:379–392. - PubMed
-
- Bowes G, Salvucci ME. Plasticity in the photosynthetic carbon metabolism of submersed aquatic macrophytes. Aquat Bot. 1989;34:232–266.
-
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

