Bundle sheath diffusive resistance to CO(2) and effectiveness of C(4) photosynthesis and refixation of photorespired CO(2) in a C(4) cycle mutant and wild-type Amaranthus edulis
- PMID: 12376660
- PMCID: PMC166622
- DOI: 10.1104/pp.008201
Bundle sheath diffusive resistance to CO(2) and effectiveness of C(4) photosynthesis and refixation of photorespired CO(2) in a C(4) cycle mutant and wild-type Amaranthus edulis
Erratum in
- Plant Physiol. 2003 May;132(1):400
Abstract
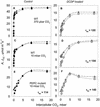
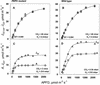
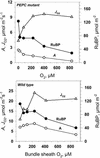
A mutant of the NAD-malic enzyme-type C(4) plant, Amaranthus edulis, which lacks phosphoenolpyruvate carboxylase (PEPC) in the mesophyll cells was studied. Analysis of CO(2) response curves of photosynthesis of the mutant, which has normal Kranz anatomy but lacks a functional C(4) cycle, provided a direct means of determining the liquid phase-diffusive resistance of atmospheric CO(2) to sites of ribulose 1,5-bisphosphate carboxylation inside bundle sheath (BS) chloroplasts (r(bs)) within intact plants. Comparisons were made with excised shoots of wild-type plants fed 3,3-dichloro-2-(dihydroxyphosphinoyl-methyl)-propenoate, an inhibitor of PEPC. Values of r(bs) in A. edulis were 70 to 180 m(2) s(-1) mol(-1), increasing as the leaf matured. This is about 70-fold higher than the liquid phase resistance for diffusion of CO(2) to Rubisco in mesophyll cells of C(3) plants. The values of r(bs) in A. edulis are sufficient for C(4) photosynthesis to elevate CO(2) in BS cells and to minimize photorespiration. The calculated CO(2) concentration in BS cells, which is dependent on input of r(bs), was about 2,000 microbar under maximum rates of CO(2) fixation, which is about six times the ambient level of CO(2). High re-assimilation of photorespired CO(2) was demonstrated in both mutant and wild-type plants at limiting CO(2) concentrations, which can be explained by high r(bs). Increasing O(2) from near zero up to ambient levels under low CO(2), resulted in an increase in the gross rate of O(2) evolution measured by chlorophyll fluorescence analysis in the PEPC mutant; this increase was simulated from a Rubisco kinetic model, which indicates effective refixation of photorespired CO(2) in BS cells.
Figures









References
-
- Brown RH. Analysis of bundle sheath conductance and C4 photosynthesis using a PEP-carboxylase inhibitor. Aust J Plant Physiol. 1997;24:549–554.
-
- Dever LV, Blackwell RD, Fullwood NJ, Lacuesta M, Leegood RC, Onek LA, Pearson M, Lea PJ. The isolation and characterization of mutants of the C4 photosynthetic pathway. J Exp Bot. 1995;46:1363–1376.
-
- Edwards GE, Baker NR. Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res. 1993;37:89–102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

