Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs
- PMID: 12376661
- PMCID: PMC166623
- DOI: 10.1104/pp.004267
Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs
Abstract
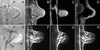
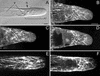
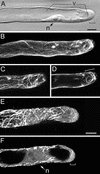
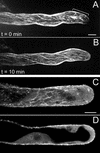
To investigate the configuration and function of microtubules (MTs) in tip-growing Medicago truncatula root hairs, we used immunocytochemistry or in vivo decoration by a GFP linked to a MT-binding domain. The two approaches gave similar results and allowed the study of MTs during hair development. Cortical MTs (CMTs) are present in all developmental stages. During the transition from bulge to a tip-growing root hair, endoplasmic MTs (EMTs) appear at the tip of the young hair and remain there until growth arrest. EMTs are a specific feature of tip-growing hairs, forming a three-dimensional array throughout the subapical cytoplasmic dense region. During growth arrest, EMTs, together with the subapical cytoplasmic dense region, progressively disappear, whereas CMTs extend further toward the tip. In full-grown root hairs, CMTs, the only remaining population of MTs, converge at the tip and their density decreases over time. Upon treatment of growing hairs with 1 microM oryzalin, EMTs disappear, but CMTs remain present. The subapical cytoplasmic dense region becomes very short, the distance nucleus tip increases, growth slows down, and the nucleus still follows the advancing tip, though at a much larger distance. Taxol has no effect on the cytoarchitecture of growing hairs; the subapical cytoplasmic dense region remains intact, the nucleus keeps its distance from the tip, but growth rate drops to the same extent as in hairs treated with 1 microM oryzalin. The role of EMTs in growing root hairs is discussed.
Figures



References
-
- Anthony RG, Hussey PJ. Dinitroaniline herbicide resistance and the microtubule cytoskeleton. Trends Plant Sci. 1999;4:112–116. - PubMed
-
- Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000;227:618–632. - PubMed
-
- Baskin TI, Miller DD, Vos JW, Wilson JE, Hepler PK. Cryofixing single cells and multicellular specimens enhances structure and immunocytochemistry for light microscopy. J Microsc. 1996;182:149–161. - PubMed
-
- Bibikova TN, Blancaflor EB, Gilroy S. Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 1999;17:657–665. - PubMed
-
- Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG. Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant-Microbe Interact. 2001;14:695–700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

