Chemical inactivation of the cinnamate 4-hydroxylase allows for the accumulation of salicylic acid in elicited cells
- PMID: 12376665
- PMCID: PMC166627
- DOI: 10.1104/pp.004309
Chemical inactivation of the cinnamate 4-hydroxylase allows for the accumulation of salicylic acid in elicited cells
Abstract
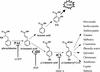
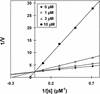
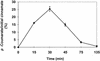
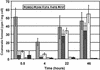
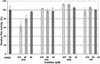
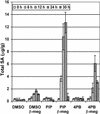
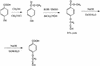
The cinnamate (CA) 4-hydroxylase (C4H) is a cytochrome P450 that catalyzes the second step of the main phenylpropanoid pathway, leading to the synthesis of lignin, pigments, and many defense molecules. Salicylic acid (SA) is an essential trigger of plant disease resistance. Some plant species can synthesize SA from CA by a mechanism not yet understood. A set of specific inhibitors of the C4H, including competitive, tight-binding, mechanism-based irreversible, and quasi-irreversible inhibitors have been developed with the main objective to redirect cinnamic acid to the synthesis of SA. Competitive inhibitors such as 2-hydroxy-1-naphthoic acid and the heme-coordinating compound 3-(4-pyridyl)-acrylic acid allowed strong inhibition of C4H activity in a tobacco (Nicotiana tabacum cv Bright Yellow [BY]) cell suspension culture. This inhibition was however rapidly relieved either because of substrate accumulation or because of inhibitor metabolism. Substrate analogs bearing a methylenedioxo function such as piperonylic acid (PIP) or a terminal acetylene such as 4-propynyloxybenzoic acid (4PB), 3-propynyloxybenzoic acid, and 4-propynyloxymethylbenzoic acid are potent mechanism-based inactivators of the C4H. PIP and 4PB, the best inactivators in vitro, were also efficient inhibitors of the enzyme in BY cells. Inhibition was not reversed 46 h after cell treatment. Cotreatment of BY cells with the fungal elicitor beta-megaspermin and PIP or 4PB led to a dramatic increase in SA accumulation. PIP and 4PB do not trigger SA accumulation in nonelicited cells in which the SA biosynthetic pathway is not activated. Mechanism-based C4H inactivators, thus, are promising tools for the elucidation of the CA-derived SA biosynthetic pathway and for the potentiation of plant defense.
Figures


References
-
- Amrhein N, Frank G, Lemm G, Luhmann HB. Inhibition of lignin formation by l-alpha-aminooxy-beta-phenylpropionic acid, an inhibitor of phenylalanine ammonia-lyase. Eur J Cell Biol. 1983;29:139–144. - PubMed
-
- Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S. A new elicitor of the hypersensitive response in tobacco: a fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995;8:551–560. - PubMed
-
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

