Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-alpha by gallbladder epithelial cells: response to bacterial lipopolysaccharides
- PMID: 12377103
- PMCID: PMC130965
- DOI: 10.1186/1471-230x-2-23
Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-alpha by gallbladder epithelial cells: response to bacterial lipopolysaccharides
Abstract
Background: In addition to immune cells, many other cell types are known to produce cytokines. Cultured normal mouse gallbladder epithelial cells, used as a model system for gallbladder epithelium, were examined for their ability to express the mRNA of various cytokines and chemokines in response to bacterial lipopolysaccharide. The synthesis and secretion of the tumor necrosis factor-alpha (TNF-alpha) protein by these cells was also measured.
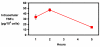
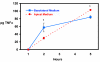
Results: Untreated mouse gallbladder cells expressed mRNA for TNF-alpha, RANTES, and macrophage inflammatory protein-2 (MIP-2). Upon treatment with lipopolysaccharide, these cells now produced mRNA for Interleukin-1beta (IL-1beta), IL-6, monocyte chemoattractant protein-1 (MCP-1), and showed increased expression of TNF-alpha and MIP-2 mRNA. Untreated mouse gallbladder cells did not synthesize TNF-alpha protein; however, they did synthesize and secrete TNF-alpha upon treatment with lipopolysaccharide.
Methods: Cells were treated with lipopolysaccharides from 3 strains of bacteria. Qualitative and semi-quantitative RT-PCR, using cytokine or chemokine-specific primers, was used to measure mRNA levels of TNFalpha, IL-1beta, IL-6, IL-10, KC, RANTES, MCP-1, and MIP-2. TNF-alpha protein was measured by immunoassays.
Conclusion: This research demonstrates that gallbladder epithelial cells in response to lipopolysaccharide exposure can alter their cytokine and chemokine RNA expression pattern and can synthesize and secrete TNFalpha protein. This suggests a mechanism whereby gallbladder epithelial cells in vivo may mediate gallbladder secretory function, inflammation and diseases in an autocrine/paracrine fashion by producing and secreting cytokines and/or chemokines during sepsis.
Figures




Similar articles
-
Proinflammatory cytokines induce RANTES and MCP-1 synthesis in human corneal keratocytes but not in corneal epithelial cells. Beta-chemokine synthesis in corneal cells.Invest Ophthalmol Vis Sci. 1996 May;37(6):987-96. Invest Ophthalmol Vis Sci. 1996. PMID: 8631642
-
Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis.J Rheumatol. 1999 Apr;26(4):791-9. J Rheumatol. 1999. PMID: 10229398
-
Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3alpha/CCL20 is induced by inflammatory cytokines.Exp Hematol. 2006 Jan;34(1):19-26. doi: 10.1016/j.exphem.2005.09.012. Exp Hematol. 2006. PMID: 16413387
-
Responses to Ang II (Angiotensin II), Salt Intake, and Lipopolysaccharide Reveal the Diverse Actions of TNF-α (Tumor Necrosis Factor-α) on Blood Pressure and Renal Function.Hypertension. 2022 Dec;79(12):2656-2670. doi: 10.1161/HYPERTENSIONAHA.122.19464. Epub 2022 Sep 21. Hypertension. 2022. PMID: 36129177 Free PMC article. Review.
-
Chemokines--their role in immunotherapy for intraocular inflammation.Ocul Immunol Inflamm. 2003 Jun;11(2):83-90. doi: 10.1076/ocii.11.2.83.15917. Ocul Immunol Inflamm. 2003. PMID: 14533027 Review.
Cited by
-
Paclitaxel interrupts TGF-beta1 signaling between gallbladder epithelial cells and myofibroblasts.J Surg Res. 2007 Aug;141(2):183-91. doi: 10.1016/j.jss.2006.12.558. Epub 2007 Jun 14. J Surg Res. 2007. PMID: 17574589 Free PMC article.
-
Volumetric Analysis of Gallbladder in Extremely Premature Infants.J Med Ultrasound. 2017 Jul-Sep;25(3):138-144. doi: 10.1016/j.jmu.2017.03.004. Epub 2017 Mar 31. J Med Ultrasound. 2017. PMID: 30065478 Free PMC article.
-
Early production of type I interferon during West Nile virus infection: role for lymphoid tissues in IRF3-independent interferon production.J Virol. 2007 Sep;81(17):9100-8. doi: 10.1128/JVI.00316-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567689 Free PMC article.
-
Cytokine single nucleotide polymorphisms in patients' with gallstone: dose TGF-β gene variants affect gallstone formation?Mol Biol Rep. 2013 Nov;40(11):6256-60. doi: 10.1007/s11033-013-2737-6. Mol Biol Rep. 2013. PMID: 24078093
-
Roles of infection, inflammation, and the immune system in cholesterol gallstone formation.Gastroenterology. 2009 Feb;136(2):425-40. doi: 10.1053/j.gastro.2008.12.031. Epub 2008 Dec 25. Gastroenterology. 2009. PMID: 19109959 Free PMC article. Review.
References
-
- Jansson R, Svanvik J. Effects of intravenous secretin and cholecystokinin on gallbladder net water absorption and motility in the cat. Gastroenterology. 1977;72:639–643. - PubMed
-
- Cho WK, Mennone A, Rydberg SA, Boyer JL. Bombesin stimulates bicarbonate secretion from rat cholangiocytes: implications for neural regulation of bile secretion. Gastroenterology. 1997;113:311–321. - PubMed
-
- Sand J, Tainio H, Nordback I. Neuropeptides in pig sphincter of Oddi, bile duct, gallbladder, and duodenum. Dig Dis Sci. 1993;38:694–700. - PubMed
-
- Elsing C, Kassner A, Stremmel W. Sodium, hydrogen antiporter activation by extracellular adenosine triphosphate in biliary epithelial cells. Gastroenterology. 1996;111:1321–1332. - PubMed
-
- McGill JM, Basavappa S, Mangel AW, Shimokura GH, Middleton JP, Fitz JG. Adenosine triphosphate activates ion permeabilities in biliary epithelial cells. Gastroenterology. 1994;107:236–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

