DNA-PKcs subunits in radiosensitization by hyperthermia on hepatocellular carcinoma hepG2 cell line
- PMID: 12378618
- PMCID: PMC4656564
- DOI: 10.3748/wjg.v8.i5.797
DNA-PKcs subunits in radiosensitization by hyperthermia on hepatocellular carcinoma hepG2 cell line
Abstract
Aim: To investigate the role of DNA-PKcs subunits in radiosensitization by hyperthermia on hepatocellular carcinoma HepG(2) cell lines.
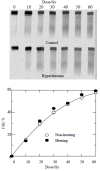
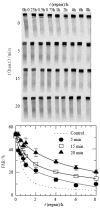
Methods: Hep G(2) cells were exposed to hyperthermia and irradiation. Hyperthermia was given at 45.5 degrees C. Cell survival was determined by an in vitro clonogenic assay for the cells treated with or without hyperthermia at various time points. DNA DSB rejoining was measured using asymmetric field inversion gel electrophoresis (AFIGE). The DNA-PKcs activities were measured using DNA-PKcs enzyme assay system.
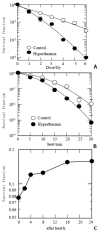
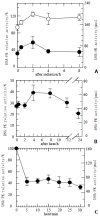
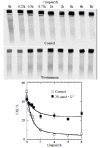
Results: Hyperthermia can significantly enhance irradiation-killing cells. Thermal enhancement ratio as calculated at 10 % survival was 2.02. The difference in radiosensitivity between two treatment modes manifested as a difference in the alpha components and the almost same beta components, which alpha value was considerably higher in the cells of combined radiation and hyperthermia as compared with irradiating cells (1.07 Gy(-1) versus 0.44 Gy(-1)). Survival fraction showed 1 logarithm increase after an 8-hour interval between heat and irradiation, whereas DNA-PKcs activity did not show any recovery. The cells were exposed to heat 5 minutes only, DNA-PKcs activity was inhibited at the nadir, even though the exposure time was lengthened. Whereas the ability of DNA DSB rejoining was inhibited with the increase of the length of hyperthermic time. The repair kinetics of DNA DSB rejoining after treatment with Wortmannin is different from the hyperthermic group due to the striking high slow rejoining component.
Conclusion: Determination with the cell extracts and the peptide phosphorylation assay, DNA-PKcs activity was inactivated by heat treatment at 45.5 degrees C, and could not restore. Cell survival is not associated with the DNA-PKcs inactivity after heat. DNA-PKcs is not a unique factor affecting the DNA DSB repair. This suggests that DNA-PKcs do not play a crucial role in the enhancement of cellular radiosensitivity by hyperthermia.
Figures
Similar articles
-
Role of DNA-PK subunits in radiosensitization by hyperthermia.Radiat Res. 1999 Aug;152(2):214-8. Radiat Res. 1999. PMID: 10409332
-
DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus.Cancer Res. 2000 Mar 1;60(5):1245-53. Cancer Res. 2000. PMID: 10728683
-
Selective inactivation of DNA-dependent protein kinase with antisense oligodeoxynucleotides: consequences for the rejoining of radiation-induced DNA double-strand breaks and radiosensitivity of human cancer cell lines.Cancer Res. 2002 Nov 15;62(22):6621-4. Cancer Res. 2002. PMID: 12438258
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
-
DNA-PKcs: A promising therapeutic target in human hepatocellular carcinoma?DNA Repair (Amst). 2016 Nov;47:12-20. doi: 10.1016/j.dnarep.2016.10.004. Epub 2016 Oct 15. DNA Repair (Amst). 2016. PMID: 27789167 Review.
Cited by
-
Effect of staurosporine on cycle of human gastric cancer cells.World J Gastroenterol. 2004 Jan 15;10(2):161-6. doi: 10.3748/wjg.v10.i2.161. World J Gastroenterol. 2004. PMID: 14716814 Free PMC article.
-
Growth arrest and apoptosis of human hepatocellular carcinoma cells induced by hexamethylene bisacetamide.World J Gastroenterol. 2004 Apr 1;10(7):954-8. doi: 10.3748/wjg.v10.i7.954. World J Gastroenterol. 2004. PMID: 15052673 Free PMC article.
-
High-biologically effective dose palliative radiotherapy for a tumor thrombus might improve the long-term prognosis of hepatocellular carcinoma: a retrospective study.Radiat Oncol. 2017 May 31;12(1):92. doi: 10.1186/s13014-017-0831-y. Radiat Oncol. 2017. PMID: 28569169 Free PMC article.
-
KU-0060648 inhibits hepatocellular carcinoma cells through DNA-PKcs-dependent and DNA-PKcs-independent mechanisms.Oncotarget. 2016 Mar 29;7(13):17047-59. doi: 10.18632/oncotarget.7742. Oncotarget. 2016. PMID: 26933997 Free PMC article.
-
Immunogenic Effect of Hyperthermia on Enhancing Radiotherapeutic Efficacy.Int J Mol Sci. 2018 Sep 17;19(9):2795. doi: 10.3390/ijms19092795. Int J Mol Sci. 2018. PMID: 30227629 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

