Azithromycin in a triple therapy for H.pylori eradication in active duodenal ulcer
- PMID: 12378634
- PMCID: PMC4656579
- DOI: 10.3748/wjg.v8.i5.879
Azithromycin in a triple therapy for H.pylori eradication in active duodenal ulcer
Abstract
Aim: To assess and compare the efficacy and safety of two triple regimes: A) metronidazole, amoxicillin and omeprazole, which is still widely used in Russia, and B) azithromycin, amoxicillin and omeprazole in healing active duodenal ulcer and H.pylori eradication.
Methods: 100 patients with active duodenal ulcer were included in the open, multicentre, randomized study with comparative groups. Patients were randomly assigned to one of the following one-week triple regimes: A) metronidazole 500 mg bid, amoxicillin 1 g bid and omeprazole 20 mg bid (OAM, n=50) and B) azithromycin 1 g od for the first 3 days (total dose 3 g), amoxicillin 1 g bid and omeprazole 20 mg bid (OAA, n=50). Omeprazole 20 mg od was given after the eradication course as a monotherapy for three weeks. The control endoscopy was performed 8 weeks after the entry. H.pylori infection was determined in the entry of the study and four weeks after the cessation of treatment by means of histology and CLO-test.
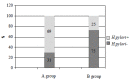
Results: 97 patients completed the study according to the protocol (1 patient of the OAM group did not come to the control endoscopy, 2 patients of the OAA group stopped the treatment because of mild allergic urticaria). Duodenal ulcers were healed in 48 patients of the OAM group (96 %; CI 90.5-100 %) and in 46 patients of the OAA group (92 %; CI 89.5-94.5 %) (p=ns). H.pylori infection was eradicated in 15 out of 50 patients with OAM (30 %; CI 17-43 %) and in 36 out of 50 patients treated with OAA (72 %; CI 59-85 %) (P<0.001)- ITT analysis.
Conclusion: The triple therapy with omeprazole, amoxicillin and metronidazole failed to eradicate H.pylori in the majority of patients, which is an essential argument to withdraw this regimen out of the national recommendations. Macrolide with amoxicillin are preferable to achieve higher eradication rates. Azithromycin (1 g od for the first 3 days) can be considered as a successful component of the triple PPI-based regimen.
Figures
References
-
- Harrison JD, Jones JA, Morris DL. Azithromycin levels in plasma and gastric tissue, juice and mucus. Eur J Clin Microbiol Infect Dis. 1991;10:862–864. - PubMed
-
- Bertoni G, Sassatelli R, Nigrisoli E, Tansini P, Bianchi G, Della Casa G, Bagni A, Bedogni G. Triple therapy with azithromycin, omeprazole, and amoxicillin is highly effective in the eradication of Helicobacter pylori: a controlled trial versus omeprazole plus amoxicillin. Am J Gastroenterol. 1996;91:258–263. - PubMed
-
- Labenz J, Tillenburg B, Stolte M. Azithromycin as a substitute for clarithromycin in French triple therapy for Helicobacter pylori: a randomized study (abstr.) Gut. 1999;45(Suppl. III):A115.
-
- Di Mario F, Dal Bó N, Grassi SA, Rugge M, Cassaro M, Donisi PM, Vianello F, Kusstatscher S, Salandin S, Grasso GA, et al. Azithromycin for the cure of Helicobacter pylori infection. Am J Gastroenterol. 1996;91:264–267. - PubMed
-
- Vcev A, Stimac D, Vceva A, Takac B, Pezerovíc D, Ivandíc A. High dose omeprazole plus amoxicillin and azithromycin in eradication of Helicobacter pylori in duodenal ulcers. Helicobacter. 1999;4:54–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous