Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms
- PMID: 12379713
- PMCID: PMC130380
- DOI: 10.1128/IAI.70.11.6339-6345.2002
Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms
Abstract
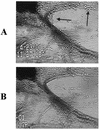
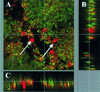
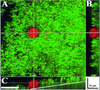
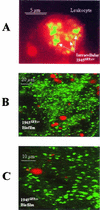
Staphylococcus aureus is a common pathogen responsible for nosocomial and community infections. It readily colonizes indwelling catheters, forming microbiotic communities termed biofilms. S. aureus bacteria in biofilms are protected from killing by antibiotics and the body's immune system. For years, one mechanism behind biofilm resistance to attack from the immune system's sentinel leukocytes has been conceptualized as a deficiency in the ability of the leukocytes to penetrate the biofilm. We demonstrate here that under conditions mimicking physiological shear, leukocytes attach, penetrate, and produce cytokines in response to maturing and fully matured S. aureus biofilm.
Figures
References
-
- Assenmacher, M., M. Lohning, A. Scheffold, R. A. Manz, J. Schmitz, and A. Radbruch. 1998. Sequential production of IL-2, IFN-gamma and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur. J. Immunol 28:1534-1543. - PubMed
-
- Benn, M., L. H. Hagelskjaer, and M. Tvede. 1997. Infective endocarditis, 1984 through 1993: a clinical and microbiological survey. J. Intern. Med. 242:15-22. - PubMed
-
- Buxton, T. B., J. Horner, A. Hinton, and J. P. Rissing. 1987. In vivo glycocalyx expression by Staphylococcus aureus phage type 52/52A/80 in S. aureus osteomyelitis. J. Infect. Dis. 156:942-946. - PubMed
-
- Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

