Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival
- PMID: 12379717
- PMCID: PMC130419
- DOI: 10.1128/IAI.70.11.6373-6382.2002
Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival
Abstract
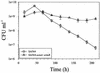
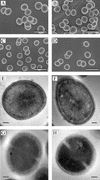
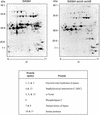
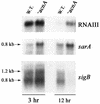
Staphylococcus aureus preferentially catabolizes glucose, generating pyruvate, which is subsequently oxidized to acetate under aerobic growth conditions. Catabolite repression of the tricarboxylic acid (TCA) cycle results in the accumulation of acetate. TCA cycle derepression coincides with exit from the exponential growth phase, the onset of acetate catabolism, and the maximal expression of secreted virulence factors. These data suggest that carbon and energy for post-exponential-phase growth and virulence factor production are derived from the catabolism of acetate mediated by the TCA cycle. To test this hypothesis, the aconitase gene was genetically inactivated in a human isolate of S. aureus, and the effects on physiology, morphology, virulence factor production, virulence for mice, and stationary-phase survival were examined. TCA cycle inactivation prevented the post-exponential growth phase catabolism of acetate, resulting in premature entry into the stationary phase. This phenotype was accompanied by a significant reduction in the production of several virulence factors and alteration in host-pathogen interaction. Unexpectedly, aconitase inactivation enhanced stationary-phase survival relative to the wild-type strain. Aconitase is an iron-sulfur cluster-containing enzyme that is highly susceptible to oxidative inactivation. We speculate that reversible loss of the iron-sulfur cluster in wild-type organisms is a survival strategy used to circumvent oxidative stress induced during host-pathogen interactions. Taken together, these data demonstrate the importance of the TCA cycle in the life cycle of this medically important pathogen.
Figures


References
-
- Babior, B. M. 2000. Phagocytes and oxidative stress. Am. J. Med. 109:33-44. - PubMed
-
- Blumenthal, H. J. 1972. Glucose catabolism in staphylococci, p. 111-135. In J. O. Cohen (ed.), The staphylococci. Wiley-Interscience, New York, N.Y.
-
- Bruckner, R. 1997. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151:1-8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

