Babesia bovis merozoite surface antigen 2 proteins are expressed on the merozoite and sporozoite surface, and specific antibodies inhibit attachment and invasion of erythrocytes
- PMID: 12379726
- PMCID: PMC130353
- DOI: 10.1128/IAI.70.11.6448-6455.2002
Babesia bovis merozoite surface antigen 2 proteins are expressed on the merozoite and sporozoite surface, and specific antibodies inhibit attachment and invasion of erythrocytes
Abstract
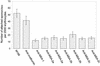
The Babesia bovis merozoite surface antigen 2 (MSA-2) locus encodes four proteins, MSA-2a(1), -2a(2), -2b, and -2c. With the use of specific antibodies, each MSA-2 protein was shown to be expressed on the surface of live extracellular merozoites and coexpression on single merozoites was confirmed. Individual antisera against MSA-2a, MSA-2b, and MSA-2c significantly inhibited merozoite invasion of bovine erythrocytes. As tick-derived sporozoites also directly invade erythrocytes, expression of each MSA-2 protein on the sporozoite surface was examined and verified. Finally, statistically significant inhibition of sporozoite binding to the erythrocytes was demonstrated by using antisera specific for MSA-2a, MSA-2b, and MSA-2c. These results indicate an important role for MSA-2 proteins in the initial binding and invasion of host erythrocytes and support the hypothesis that sporozoites and merozoites use common surface molecules in erythrocyte invasion.
Figures

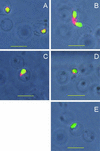
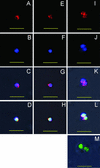
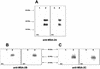
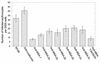

References
-
- Brown, W. C., and G. H. Palmer. 1999. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 15:275-281. - PubMed
-
- Chitnis, C. E., and M. J. Blackman. 2000. Host cell invasion by malaria parasites. Parasitol. Today 16:411-415. - PubMed
-
- Cowman, A. F., D. L. Baldi, M. Duraisingh, J. Healer, K. E. Mills, R. A. O'Donnell, J. Thompson, T. Triglia, M. E. Wickham, and B. S. Crabb. 2002. Functional analysis of Plasmodium falciparum merozoite antigens: implications for erythrocyte invasion and vaccine development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357:25-33. - PMC - PubMed
-
- Egan, A. F., P. Burghaus, P. Druilhe, A. A. Holder, and E. M. Riley. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

