RB activation defect in tumor cell lines
- PMID: 12379745
- PMCID: PMC137861
- DOI: 10.1073/pnas.212519499
RB activation defect in tumor cell lines
Abstract
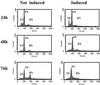
Activation of the retinoblastoma (RB) protein through dephosphorylation arises in cells upon exit from M phase and in response to environmental stresses, including DNA damage. We provide here for the first time evidence that these responses are co-ordinately affected in a subset of tumor derived cell lines. We find that RB dephosphorylation is not apparent in these cells during progression into G(1). Importantly these cells also do not respond with RB activation after DNA damage during S phase. Moreover and as a consequence they display phenotypes classically associated with RB(-) cells, showing accelerated apoptosis after DNA damage and DNA re-replication after spindle-checkpoint activation. A large body of literature provides evidence that controls governing inactivation of RB are lost in tumors. The results presented here indicate that the reverse reaction, namely the activation of RB from an inactive precursor, may also be compromised. Our findings indicate that this type of defect may be coupled with hypersensitivity to DNA damage and an increase in genomic instability in response to spindle-checkpoint activation thus bearing potentially important medical implications.
Figures



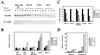
References
-
- Zheng L. & Lee, W. H. (2001) Exp. Cell Res. 264, 2-18. - PubMed
-
- Morris E. J. & Dyson, N. J. (2001) Adv. Cancer Res. 82, 1-54. - PubMed
-
- Mittnacht S. (1998) Curr. Opin. Genet. Dev. 8, 21-27. - PubMed
-
- Adams P. D. (2001) Biochim. Biophys. Acta 1471, M123-M133. - PubMed
-
- Harbour J. W. & Dean, D. C. (2000) Genes Dev. 14, 2393-2409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

