Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts
- PMID: 12379806
- PMCID: PMC2173484
- DOI: 10.1083/jcb.200204046
Insulin receptor substrate-2 maintains predominance of anabolic function over catabolic function of osteoblasts
Abstract
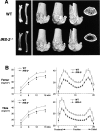
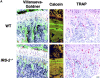
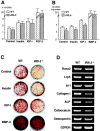
Insulin receptor substrates (IRS-1 and IRS-2) are essential for intracellular signaling by insulin and insulin-like growth factor-I (IGF-I), anabolic regulators of bone metabolism. Although mice lacking the IRS-2 gene (IRS-2-/- mice) developed normally, they exhibited osteopenia with decreased bone formation and increased bone resorption. Cultured IRS-2-/- osteoblasts showed reduced differentiation and matrix synthesis compared with wild-type osteoblasts. However, they showed increased receptor activator of nuclear factor kappaB ligand (RANKL) expression and osteoclastogenesis in the coculture with bone marrow cells, which were restored by reintroduction of IRS-2 using an adenovirus vector. Although IRS-2 was expressed and phosphorylated by insulin and IGF-I in both osteoblasts and osteoclastic cells, cultures in the absence of osteoblasts revealed that intrinsic IRS-2 signaling in osteoclastic cells was not important for their differentiation, function, or survival. It is concluded that IRS-2 deficiency in osteoblasts causes osteopenia through impaired anabolic function and enhanced supporting ability of osteoclastogenesis. We propose that IRS-2 is needed to maintain the predominance of bone formation over bone resorption, whereas IRS-1 maintains bone turnover, as we previously reported; the integration of these two signalings causes a potent bone anabolic action by insulin and IGF-I.
Figures

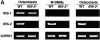

References
-
- Akune, T., N. Ogata, A. Seichi, I. Ohnishi, K. Nakamura, and H. Kawaguchi. 2001. Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J. Bone Joint Surg. Am. 83:1537–1544. - PubMed
-
- Araki, E., M.A. Lipes, M.E. Patti, J.C. Bruning, B. Haag, III, R.S. Johnson, and C.R. Kahn. 1994. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature. 372:186–190. - PubMed
-
- Breyer, R.M., C.K. Bagdassarian, S.A. Myers, and M.D. Breyer. 2001. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41:661–690. - PubMed
-
- Burks, D.J., and M.F. White. 2001. IRS proteins and β-cell function. Diabetes. 50:S140–S145. - PubMed

