Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation
- PMID: 12380690
- PMCID: PMC514820
- DOI: 10.1379/1466-1268(2002)007<0213:vstroe>2.0.co;2
Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation
Abstract
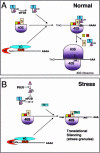
Eukaryotic cells express a family of eukaryotic translation initiation factor 2 alpha (eIF2alpha) kinases (eg, PKR, PERK-PEK, GCN2, HRI) that are individually activated in response to distinct types of environmental stress. Phosphorylation of eIF2alpha by one or more of these kinases reduces the concentration of eIF2-guanosine triphosphate (GTP)-transfer ribonucleic acid for methionine (tRNA(Met)), the ternary complex that loads tRNA(Met) onto the small ribosomal subunit to initiate protein translation. When ternary complex levels are reduced, the related RNA-binding proteins TIA-1 and TIAR promote the assembly of a noncanonical preinitiation complex that lacks eIF2-GTP-tRNA(Met). The TIA proteins dynamically sort these translationally incompetent preinitiation complexes into discrete cytoplasmic domains known as stress granules (SGs). RNA-binding proteins that stabilize or destabilize messenger RNA (mRNA) are also recruited to SGs during stress. Thus, TIA-1 and TIAR act downstream of eIF2alpha phosphorylation to promote SG assembly and facilitate mRNA triage during stress. The role of the SG in the integration of translational efficiency, mRNA stability, and the stress response is discussed.
Figures

References
-
- Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
