Building consensus on nomenclature and disease classification for ankylosing spondylitis: results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis, Berlin, Germany, 18-19 January 2002
- PMID: 12381512
- PMCID: PMC1766727
- DOI: 10.1136/ard.61.suppl_3.iii61
Building consensus on nomenclature and disease classification for ankylosing spondylitis: results and discussion of a questionnaire prepared for the International Workshop on New Treatment Strategies in Ankylosing Spondylitis, Berlin, Germany, 18-19 January 2002
Abstract
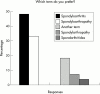
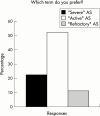
Background: There is currently no universal consensus on nomenclature for spondyloarthropathy (SpA), or on activity and severity criteria for ankylosing spondylitis (AS).
Method: Points of agreement and majority opinions among 28 international experts in the field were identified by questionnaire. Agreement was defined as >80% concurrence, clear majority as >60% concurrence, and a majority or trend as >50% concurrence.
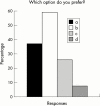
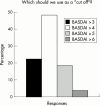
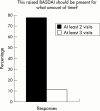
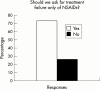
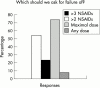
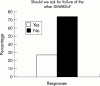
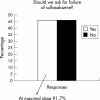
Results: Respondents agreed on the need for one term that reflects the inflammatory nature of the disease, but no agreement was reached on a specific term. Agreement included subdivision of patients with SpA into AS, psoriatic arthritis, inflammatory bowel disease associated arthritis, and undifferentiated spondyloarthritis/spondyloarthropathy. A majority of experts defined active disease as fulfilling classification criteria for AS and/or a SpA, and disease activity measured by a Bath AS Disease Activity Index (BASDAI) score >4 determined by two patient visits during a two month period, but no maximum radiographic score. The majority of participants considered failure of treatment response to non-steroidal anti-inflammatory drugs (NSAIDs) alone to be a prerequisite for active/severe AS, and 15/28 (54%) thought that NSAID treatment failure should be defined as lack of response to two or more NSAIDs.
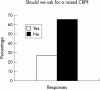
Conclusions: Respondents agreed that a two to five year study is the ethical method to demonstrate effects of anti-tumour necrosis factor alpha (TNFalpha) therapy on radiographic progression of AS, and that inclusion criteria should include a certain level of disease activity (measured by BASDAI) and failure of certain treatments. After the efficacy of anti-TNFalpha therapy in AS and psoriatic arthritis is proved, respondents agreed that more studies will be needed to show efficacy for other SpA subsets.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

