Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis
- PMID: 12381667
- PMCID: PMC187458
- DOI: 10.1101/gad.242002
Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis
Abstract
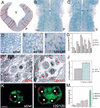
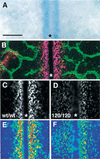
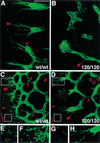
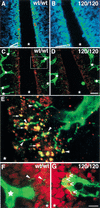
Branching morphogenesis in the mammalian lung and Drosophila trachea relies on the precise localization of secreted modulators of epithelial growth to select branch sites and direct branch elongation, but the intercellular signals that control blood vessel branching have not been previously identified. We found that VEGF(120/120) mouse embryos, engineered to express solely an isoform of VEGF-A that lacks heparin-binding, and therefore extracellular matrix interaction domains, exhibited a specific decrease in capillary branch formation. This defect was not caused by isoform-specific differences in stimulating endothelial cell proliferation or by impaired isoform-specific signaling through the Nrp1 receptor. Rather, changes in the extracellular localization of VEGF-A in heparin-binding mutant embryos resulted in an altered distribution of endothelial cells within the growing vasculature. Instead of being recruited into additional branches, nascent endothelial cells were preferentially integrated within existing vessels to increase lumen caliber. The disruption of the normal VEGF-A concentration gradient also impaired the directed extension of endothelial cell filopodia, suggesting that heparin-binding VEGF-A isoforms normally provide spatially restricted stimulatory cues that polarize and thereby guide sprouting endothelial cells to initiate vascular branch formation. Consistent with this idea, we found opposing defects in embryos harboring only a heparin-binding isoform of VEGF-A, including excess endothelial filopodia and abnormally thin vessel branches in ectopic sites. We conclude that differential VEGF-A isoform localization in the extracellular space provides a control point for regulating vascular branching pattern.
Figures


References
-
- Abrahamson DR, Robert B, Hyink DP, St. John PL, Daniel TO. Origins and formation of microvasculature in the developing kidney. Kidney Int Suppl. 1998;67:S7–S11. - PubMed
-
- Baeg GH, Perrimon N. Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila. Curr Opin Cell Biol. 2000;12:575–580. - PubMed
-
- Bär T. Patterns of vascularization in the developing cerebral cortex. CIBA Found Symp. 1983;100:20–36. - PubMed
-
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
