Characterization of K(+) currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans
- PMID: 12381812
- PMCID: PMC2290601
- DOI: 10.1113/jphysiol.2002.022293
Characterization of K(+) currents using an in situ patch clamp technique in body wall muscle cells from Caenorhabditis elegans
Abstract
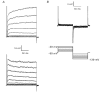
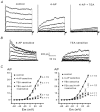
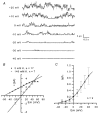
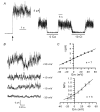
The properties of K(+) channels in body wall muscle cells acutely dissected from the nematode Caenorhabditis elegans were investigated at the macroscopic and unitary level using an in situ patch clamp technique. In the whole-cell configuration, depolarizations to potentials positive to -40 mV gave rise to outward currents resulting from the activation of two kinetically distinct voltage-dependent K(+) currents: a fast activating and inactivating 4-aminopyridine-sensitive component and a slowly activating and maintained tetraethylammonium-sensitive component. In cell-attached patches, voltage-dependent K(+) channels, with unitary conductances of 34 and 80 pS in the presence of 5 and 140 mM external K(+), respectively, activated at membrane potentials positive to -40 mV. Excision revealed that these channels corresponded to Ca(2+)-activated K(+) channels exhibiting an unusual sensitivity to internal Cl(-) and whose activity progressively decreased in inside-out conditions. After complete run-down of these channels, one third of inside-out patches displayed activity of another Ca(2+)-activated K(+) channel of smaller unitary conductance (6 pS at 0 mV in the presence of 5 mM external K(+)). In providing a detailed description of native K(+) currents in body wall muscle cells of C. elegans, this work lays the basis for further comparisons with mutants to assess the function of K(+) channels in this model organism that is highly amenable to molecular and classical genetics.
Figures

Similar articles
-
Ionic currents in single smooth muscle cells of the canine renal artery.Circ Res. 1992 Oct;71(4):745-58. doi: 10.1161/01.res.71.4.745. Circ Res. 1992. PMID: 1381293
-
Two types of voltage-dependent potassium channels in outer hair cells from the guinea pig cochlea.Am J Physiol. 1999 Nov;277(5):C913-25. doi: 10.1152/ajpcell.1999.277.5.C913. Am J Physiol. 1999. PMID: 10564084
-
Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons.J Neurophysiol. 2000 Jan;83(1):70-80. doi: 10.1152/jn.2000.83.1.70. J Neurophysiol. 2000. PMID: 10634854
-
A patch-clamp study of K(+)-channel activity in bovine isolated tracheal smooth muscle cells.Br J Pharmacol. 1991 Apr;102(4):871-8. doi: 10.1111/j.1476-5381.1991.tb12269.x. Br J Pharmacol. 1991. PMID: 1713110 Free PMC article.
-
Block of large conductance Ca(2+)-activated K+ channels in rabbit vascular myocytes by internal Mg2+ and Na+.J Physiol. 1996 Sep 15;495 ( Pt 3)(Pt 3):701-16. doi: 10.1113/jphysiol.1996.sp021627. J Physiol. 1996. PMID: 8887777 Free PMC article.
Cited by
-
Mutant analysis of the Shal (Kv4) voltage-gated fast transient K+ channel in Caenorhabditis elegans.J Biol Chem. 2006 Oct 13;281(41):30725-35. doi: 10.1074/jbc.M605814200. Epub 2006 Aug 9. J Biol Chem. 2006. PMID: 16899454 Free PMC article.
-
Patch clamp study of the UNC-105 degenerin and its interaction with the LET-2 collagen in Caenorhabditis elegans muscle.J Physiol. 2004 Jun 1;557(Pt 2):379-88. doi: 10.1113/jphysiol.2003.057687. Epub 2004 Mar 12. J Physiol. 2004. PMID: 15020702 Free PMC article.
-
Emodepside and SL0-1 potassium channels: a review.Exp Parasitol. 2012 Sep;132(1):40-6. doi: 10.1016/j.exppara.2011.08.012. Epub 2011 Sep 3. Exp Parasitol. 2012. PMID: 21910990 Free PMC article. Review.
-
Dissection of K+ currents in Caenorhabditis elegans muscle cells by genetics and RNA interference.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14391-6. doi: 10.1073/pnas.1935976100. Epub 2003 Nov 11. Proc Natl Acad Sci U S A. 2003. PMID: 14612577 Free PMC article.
-
K+ Accumulation and Clearance in the Calyx Synaptic Cleft of Type I Mouse Vestibular Hair Cells.Neuroscience. 2020 Feb 1;426:69-86. doi: 10.1016/j.neuroscience.2019.11.028. Epub 2019 Dec 14. Neuroscience. 2020. PMID: 31846752 Free PMC article.
References
-
- Adams DJ, Nonner W. Voltage-dependent potassium channels: gating, ion permeation and block. In: Cook NS, editor. Potassium Channels, Structure, Classification, Function and Therapeutic Potential. Chichester, UK: Ellis Horwood Limited; 1990. pp. 40–69.
-
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

