Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels
- PMID: 12381816
- PMCID: PMC2290605
- DOI: 10.1113/jphysiol.2002.022434
Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels
Abstract
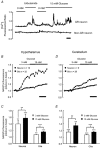
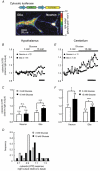
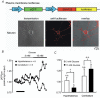
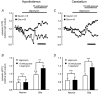
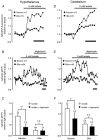
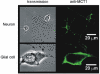
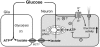
Glucose-responsive (GR) neurons from hypothalamic nuclei are implicated in the regulation of feeding and satiety. To determine the role of intracellular ATP in the closure of ATP-sensitive K(+) (K(ATP)) channels in these cells and associated glia, the cytosolic ATP concentration ([ATP](c)) was monitored in vivo using adenoviral-driven expression of recombinant targeted luciferases and bioluminescence imaging. Arguing against a role for ATP in the closure of K(ATP) channels in GR neurons, glucose (3 or 15 mM) caused no detectable increase in [ATP](c), monitored with cytosolic luciferase, and only a small decrease in the concentration of ATP immediately beneath the plasma membrane, monitored with a SNAP25-luciferase fusion protein. In contrast to hypothalamic neurons, hypothalamic glia responded to glucose (3 and 15 mM) with a significant increase in [ATP](c). Both neurons and glia from the cerebellum, a glucose-unresponsive region of the brain, responded robustly to 3 or 15 mM glucose with increases in [ATP](c). Further implicating an ATP-independent mechanism of K(ATP) channel closure in hypothalamic neurons, removal of extracellular glucose (10 mM) suppressed the electrical activity of GR neurons in the presence of a fixed, high concentration (3 mM) of intracellular ATP. Neurons from both brain regions responded to 5 mM lactate (but not pyruvate) with an oligomycin-sensitive increase in [ATP](c). High levels of the plasma membrane lactate-monocarboxylate transporter, MCT1, were found in both cell types, and exogenous lactate efficiently closed K(ATP) channels in GR neurons. These data suggest that (1) ATP-independent intracellular signalling mechanisms lead to the stimulation of hypothalamic neurons by glucose, and (2) these effects may be potentiated in vivo by the release of lactate from neighbouring glial cells.
Figures
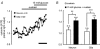


Comment in
-
Shedding new light on brain metabolism and glial function.J Physiol. 2002 Oct 15;544(Pt 2):334. doi: 10.1113/jphysiol.2002.029090. J Physiol. 2002. PMID: 12381806 Free PMC article. No abstract available.
References
-
- Ainscow EK, Zhao C, Rutter GA. Acute overexpression of lactate dehydrogenase-A perturbs β-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. - PubMed
-
- Ämmälä C, Eliasson L, Bokvist K, Berggren PO, Honkanen RE, Sjoholm A, Rorsman P. Activation of protein kinases and inhibition of protein phosphatases play a central role in the regulation of exocytosis in mouse pancreatic β cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:4343–4347. - PMC - PubMed
-
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Archiv. 1990;415:479–483. - PubMed
-
- Bergmeyer H-U. Lactate. In: Bergmeyer H-U, editor. Methods of Enzymatic Analysis. 2. New York, London: Weinheim-Verlag Chemie, Academic Press; 1965. pp. 278–282.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

