Individual mouse taste cells respond to multiple chemical stimuli
- PMID: 12381822
- PMCID: PMC2290593
- DOI: 10.1113/jphysiol.2002.027862
Individual mouse taste cells respond to multiple chemical stimuli
Abstract
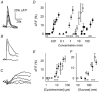
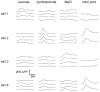
Sensory organs are specialized to detect and decode stimuli in terms of intensity and quality. In the gustatory system, the process of identifying and distinguishing taste qualities (e.g. bitter versus sweet) begins in taste buds. A central question in gustatory research is how information about taste quality is extracted by taste receptor cells. For instance, whether and how individual taste cells respond to multiple chemical stimuli is still a matter for debate. A recent study showed that taste cells expressing bitter-responsive taste receptors do not also express sweet-responsive taste receptors and vice versa. These results suggest that the gustatory system may use separate cellular pathways to process bitter and sweet signals independently. Results from electrophysiological studies, however, reveal that individual taste receptor cells respond to stimuli representing multiple taste qualities. Here we used non-invasive Ca(2+) imaging in slices of lingual tissue containing taste buds to address the issue of quality detection in murine taste receptor cells. We recorded calcium transients elicited by chemical stimuli representing different taste qualities (sweet, salty, sour and bitter). Many receptor cells (38 %) responded to multiple taste qualities, with some taste cells responding to both appetitive ("sweet") and aversive ("bitter") stimuli. Thus, there appears to be no strict and separate detection of taste qualities by distinct subpopulations of taste cells in peripheral gustatory sensory organs in mice.
Figures



References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
-
- Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

