Resolution of skeletal muscle inflammation in mdx dystrophic mouse is accompanied by increased immunoglobulin and interferon-gamma production
- PMID: 12383191
- PMCID: PMC2517677
- DOI: 10.1046/j.1365-2613.2002.00221.x
Resolution of skeletal muscle inflammation in mdx dystrophic mouse is accompanied by increased immunoglobulin and interferon-gamma production
Abstract
Mdx mouse, the animal model of Duchenne muscular dystrophy, develops an X-linked recessive inflammatory myopathy with an apparent sustained capacity for muscle regeneration. We analysed whether changes in the skeletal muscle during myonecrosis and regeneration would correlate with functional alterations in peripheral lymphoid tissues. Here we show that during the height of myonecrosis, mdx mice display marked atrophy of peripheral lymph nodes and extensive muscle inflammation. In contrast, enlargement of draining lymph nodes with accumulation of CD4+ CD44+, CD4+ CD25+, CD8+ CD44+ T lymphocytes and type-2 B cells was consistently observed during amelioration of the muscle lesion. In addition, regeneration of the muscular tissue was accompanied by concomitant increase of immunoglobulin-secreting cells in regional lymph nodes and bone marrow. Double immunolabelling analysis revealed intense B cell proliferation and formation of germinal centre in the follicles of dystrophic regional lymph nodes. Furthermore, lymph node cells produced large amounts of IFN-gamma but not IL-4, IL-6 or IL-10 after in vitro mitogen stimulation with Concanavalin A. As these alterations occurred mainly during the recovery period, we suggested that local activation of the immune system could be an influence which mitigates the myonecrosis of muscular tissue in the mdx dystrophic mouse.
Figures

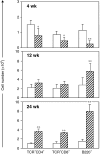
 mdx. *P < 0.05 and **P < 0.0001.
mdx. *P < 0.05 and **P < 0.0001.
 mdx. *P < 0.05.
mdx. *P < 0.05.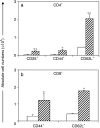
 mdx. *P < 0.05, **P < 0.0001.
mdx. *P < 0.05, **P < 0.0001.
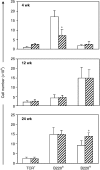
 mdx. *P < 0.05.
mdx. *P < 0.05.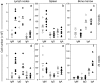
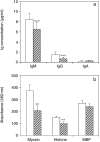
 mdx. **P < 0.001, ***P < 0.0001.
mdx. **P < 0.001, ***P < 0.0001.
 mdx. *P < 0.05, **P < 0.005.
mdx. *P < 0.05, **P < 0.005.References
-
- Allamand V, Campbell KP. Animal models for muscular dystrophy: valuable tools for the development of therapies. HumMolGenet. 2000;9:2459–2467. - PubMed
-
- Amemiya K, Semino-Mora C, Granger RP, Dalakas MC. Downregulation of TGF-β1 mRNA and protein in the muscles of patients with inflammatory myopathies after treatment with high-dose intravenous immunoglobulin. ClinImmunol. 2000;94:99–104. - PubMed
-
- Billiau A. Interferon-γ: biology and role in pathogenesis. AdvImmunol. 1996;62:61–109. - PubMed
-
- Boland B, Himpens B, Denef JF, Gillis JM. Site-dependent pathological differences in smooth muscles and skeletal muscles of the adult mdx mouse. Muscle & Nerve. 1995;18:649–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

