In vivo pharmacodynamics of a new oxazolidinone (linezolid)
- PMID: 12384354
- PMCID: PMC128755
- DOI: 10.1128/AAC.46.11.3484-3489.2002
In vivo pharmacodynamics of a new oxazolidinone (linezolid)
Abstract
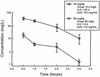
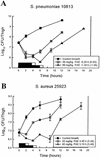
Linezolid is a new oxazolidinone with activity against gram-positive cocci. We determined the in vivo activity of linezolid against four strains of Staphylococcus aureus (two methicillin-susceptible S. aureus [MSSA] strains and two methicillin-resistant S. aureus strains) and one penicillin-susceptible Streptococcus pneumoniae (PSSP) strain, two penicillin-intermediate S. pneumoniae strains, and five penicillin-resistant S. pneumoniae strains. The mice had 10(6.3) to 10(7.7) CFU/thigh before therapy and were then treated for 24 h with 5 to 1,280 mg of linezolid/kg divided into 1, 2, 4, 8, or 16 doses. The killing activities after 4 h of therapy ranged from 2.4 to 5.0 log(10) CFU/thigh against S. pneumoniae and 1.35 to 2.2 log(10) CFU/thigh against S. aureus. Increasing doses produced minimal concentration-dependent killing; doses of 20 and 80 mg/kg produced no in vivo postantibiotic effects (PAEs) with PSSP and modest PAEs (3.4 and 3.2 h) with MSSA. Pharmacokinetic studies at doses of 20 and 80 mg/kg by high-pressure liquid chromatography analysis exhibited peak dose values of 0.68 and 0.71 and elimination half-lives of 1.02 and 1.00 h. Linezolid MICs ranged from 0.5 to 1.0 micro g/ml for S. pneumoniae and from 1.0 to 4.0 micro g/ml for S. aureus. A sigmoid dose-response model was used to estimate the dose required to achieve a net bacteriostatic effect over 24 h. Static doses against S. pneumoniae ranged from 22.2 to 97.1 mg/kg/24 h and from 133 to 167 mg/kg/24 h for S. aureus. The 24-h area under the concentration-time curve (AUC)/MIC ratio was the major parameter determining the efficacy of linezolid against PSSP (R(2) = 82% for AUC/MIC versus 57% for T>MIC and 59% for the peak level in serum/MIC [peak/MIC]). It was difficult to determine the most relevant pharmacokinetic/pharmacodynamic parameter with S. aureus, although the outcomes correlated slightly better with the 24-h AUC/MIC ratio (R(2) = 75%) than with the other parameters (T>MIC R(2) = 75% and peak/MIC R(2) = 65%). The 24-h AUC/MIC ratio required for a bacteriostatic effect with linezolid varied from 22 to 97 (mean = 48) for pneumococci and from 39 to 167 (mean = 83) for staphylococci. Based upon a pharmacokinetic goal of a 24-h AUC/MIC of 50 to 100, a dosage regimen of 600 mg given either intravenously or orally twice daily would achieve success against organisms with MICs as high as 2 to 4 micro g/ml.
Figures
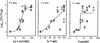
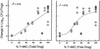

References
-
- Chien, J. W., M. L. Kucia, and R. A. Salata. 2000. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clin. Infect. Dis. 30:146-151. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. - PubMed
-
- Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
-
- Craig, W. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):55-57. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

