La autoantigen is required for the internal ribosome entry site-mediated translation of Coxsackievirus B3 RNA
- PMID: 12384597
- PMCID: PMC137146
- DOI: 10.1093/nar/gkf583
La autoantigen is required for the internal ribosome entry site-mediated translation of Coxsackievirus B3 RNA
Abstract
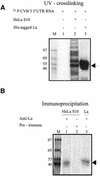
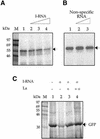
Translation initiation in Coxsackievirus B3 (CVB3) occurs via ribosome binding to an internal ribosome entry site (IRES) located in the 5'-untranslated region (UTR) of the viral RNA. This unique mechanism of translation initiation requires various trans-acting factors from the host. We show that human La autoantigen (La) binds to the CVB3 5'-UTR and also demonstrate the dose-dependent effect of exogenously added La protein in stimulating CVB3 IRES-mediated translation. The requirement of La for CVB3 IRES mediated translation has been further demonstrated by inhibition of translation as a result of sequestering La and its restoration by exogenous addition of recombinant La protein. The abundance of La protein in various mouse tissue extracts has been probed using anti-La antibody. Pancreatic tissue, a target organ for CVB3 infection, was found to have a large abundance of La protein which was demonstrated to interact with the CVB3 5'-UTR. Furthermore, exogenous addition of pancreas extract to in vitro translation reactions resulted in a dose dependent stimulation of CVB3 IRES-mediated translation. These observations indicate the role of La in CVB3 IRES-mediated translation, and suggest its possible involvement in the efficient translation of the viral RNA in the pancreas.
Figures




References
-
- Iizuka N., Chen,C., Yang,O., Johannes,G. and Sarnow,P. (1995) Cap-independent translation and internal initiation of translation in eukaryotic cellular mRNA molecules. Curr. Top. Microbiol. Immunol., 203, 155–177. - PubMed
-
- Jackson R.J., Hunt,S.L., Reynolds,J.E. and Kaminski,A. (1995) Cap-dependent and cap-independent translation: operational distinctions and mechanistic interpretations. Curr. Top. Microbiol. Immunol. 203, 1–29. - PubMed
-
- Pelletier J. and Sonenberg,N. (1988) Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature, 334, 320–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

