Oral pristinamycin versus standard penicillin regimen to treat erysipelas in adults: randomised, non-inferiority, open trial
- PMID: 12386036
- PMCID: PMC129632
- DOI: 10.1136/bmj.325.7369.864
Oral pristinamycin versus standard penicillin regimen to treat erysipelas in adults: randomised, non-inferiority, open trial
Abstract
Objective: To assess the efficacy and safety of oral pristinamycin versus intravenous then oral penicillin to treat erysipelas in patients in hospital.
Design: Multicentre, parallel group, open labelled, randomised non-inferiority trial.
Setting: 22 French hospitals.
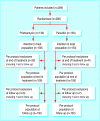
Participants: 289 adults admitted to hospital with erysipelas.
Results: At follow up (day 25-45) the cure rate (primary efficacy end point) for the per protocol populations was 81% (83/102) for pristinamycin and 67% (68/102) for penicillin. The planned interim analysis (global one sided type I error 5%) showed that the one sided 97.06% confidence interval of the observed difference (pristinamycin-penicillin) between cure rates (3.3% to infinity ) exceeded the -10% non-inferiority threshold. For the intention to treat populations the cure rate at follow up was 65% (90/138) for pristinamycin and 53% (79/150) for penicillin, with the one sided 97.06% confidence interval of the observed difference between cure rates (1.7% to infinity ) exceeding the -10% non-inferiority threshold. That the lower limit of the confidence interval exceeded the -10% threshold and was also >0 supports the hypothesis that pristinamycin is significantly superior at the 5% level. More adverse events related to treatment, as assessed by the investigators, were reported in the pristinamycin group than in the penicillin group. Most adverse events involved the gastrointestinal tract (nausea, vomiting, and diarrhoea) but were minor and usually did not require discontinuation of treatment.
Conclusion: Pristinamycin could be an alternative to the standard intravenous then oral penicillin regimen used to treat erysipelas in adults in hospital, with the advantages of oral first line therapy.
Figures
References
-
- Bernard P, Bedane C, Mounier M, Denis F, Catanzano G, Bonnetblanc JM. Streptococcal cause of erysipelas and cellulitis in adults: a microbiologic study. Arch Dermatol. 1989;125:779–782. - PubMed
-
- Duvanel T, Auckenthaler R, Rohner P, Harms M, Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149:293–296. - PubMed
-
- Chartier C, Grosshans E. Erysipelas. Int J Dermatol. 1990;29:459–467. - PubMed
-
- Swartz MN. Cellulitis and subcutaneous tissue infections. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. New York: Churchill Livingstone; 1995. pp. 909–915.
-
- Bisno AL, Stevens DL. Streptococcal infections of the skin and soft tissues. N Engl J Med. 1996;334:240–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
