Role of the lever arm in the processive stepping of myosin V
- PMID: 12386339
- PMCID: PMC137854
- DOI: 10.1073/pnas.182539599
Role of the lever arm in the processive stepping of myosin V
Abstract
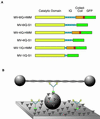
Myosin V is a two-headed molecular motor that binds six light chains per heavy chain, which creates unusually long lever arms. This motor moves processively along its actin track in discrete 36-nm steps. Our model is that one head of the two-headed myosin V tightly binds to actin and swings its long lever arm through a large angle, providing a stroke. We created single-headed constructs with different-size lever arms and show that stroke size is proportional to lever arm length. In a two-headed molecule, the stroke provides the directional bias, after which the unbound head diffuses to find its binding site, 36 nm forward. Our two-headed construct with all six light chains per head reconstitutes the 36-nm processive step seen in tissue-purified myosin V. Two-headed myosin V molecules with only four light chains per head are still processive, but their step size is reduced to 24 nm. A further reduction in the length of the lever arms to one light chain per head results in a motor that is unable to walk processively. This motor produces single small approximately 6-nm strokes, and ATPase and pyrene actin quench measurements show that only one of the heads of this dimer rapidly binds to actin for a given binding event. These data show that for myosin V with its normal proximal tail domain, both heads and a long lever arm are required for large, processive steps.
Figures




References
-
- Cheney R. E., O'Shea, M. K., Heuser, J. E., Coelho, M. V., Wolenski, J. S., Espreafico, E. M., Forscher, P., Larson, R. E. & Mooseker, M. S. (1993) Cell 75, 13-23. - PubMed
-
- Spudich J. A. (2001) Nat. Rev. Mol. Cell. Biol. 2, 387-392. - PubMed
-
- Tyska M. J. & Warshaw, D. M. (2002) Cell Motil. Cytoskeleton 51, 1-15. - PubMed
-
- Geeves M. A. & Holmes, K. C. (1999) Annu. Rev. Biochem. 68, 687-728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

