Electronic detection of DNA by its intrinsic molecular charge
- PMID: 12386345
- PMCID: PMC137851
- DOI: 10.1073/pnas.232276699
Electronic detection of DNA by its intrinsic molecular charge
Abstract
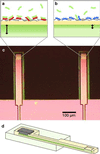
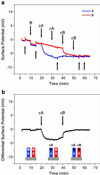
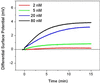
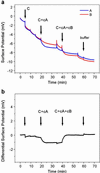
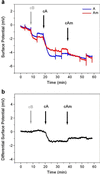
We report the selective and real-time detection of label-free DNA using an electronic readout. Microfabricated silicon field-effect sensors were used to directly monitor the increase in surface charge when DNA hybridizes on the sensor surface. The electrostatic immobilization of probe DNA on a positively charged poly-l-lysine layer allows hybridization at low ionic strength where field-effect sensing is most sensitive. Nanomolar DNA concentrations can be detected within minutes, and a single base mismatch within 12-mer oligonucleotides can be distinguished by using a differential detection technique with two sensors in parallel. The sensors were fabricated by standard silicon microtechnology and show promise for future electronic DNA arrays and rapid characterization of nucleic acid samples. This approach demonstrates the most direct and simple translation of genetic information to microelectronics.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

