Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat
- PMID: 12388587
- PMCID: PMC6757699
- DOI: 10.1523/JNEUROSCI.22-20-08808.2002
Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat
Abstract
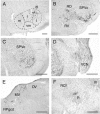
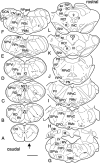
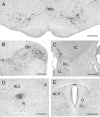
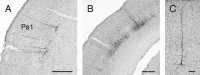
Retrograde transneuronal tracing with rabies virus from the right orbicularis oculi muscle was used to identify neural networks underlying spontaneous, reflex, and learned blinks. The kinetics of viral transfer was studied at sequential 12 hr intervals between 3 and 5 d after inoculation. Rabies virus immunolabeling was combined with the immunohistochemical detection of choline acetyltransferase expression in brainstem motoneurons or Fluoro-Ruby injections in the rubrospinal tract. Virus uptake involved exclusively orbicularis oculi motoneurons in the dorsolateral division of the facial nucleus. At 3-3.5 d, transneuronal transfer involved premotor interneurons of trigeminal, auditory, and vestibular reflex pathways (in medullary and pontine reticular formation, trigeminal nuclei, periolivary and ventral cochlear nuclei, and medial vestibular nuclei), motor pathways (dorsolateral quadrant of contralateral red nucleus and pararubral area), deep cerebellar nuclei (lateral portion of interpositus nucleus and dorsolateral hump ipsilaterally), limbic relays (parabrachial and Kölliker-Fuse nuclei), and oculomotor structures involved in eye-eyelid coordination (oculomotor nucleus, supraoculomotor area, and interstitial nucleus of Cajal). At 4 d, higher order neurons were revealed in trigeminal, auditory, vestibular, and deep cerebellar nuclei (medial, interpositus, and lateral), oculomotor and visual-related structures (Darkschewitsch, nucleus of the posterior commissure, deep layers of superior colliculus, and pretectal area), lateral hypothalamus, and cerebral cortex (particularly in parietal areas). At 4.5 and 5 d the labeling of higher order neurons occurred in hypothalamus, cerebral cortex, and blink-related areas of cerebellar cortex. These results provide a comprehensive picture of the premotor networks mediating reflex, voluntary, and limbic-related eyelid responses and highlight potential sites of motor learning in eyelid classical conditioning.
Figures






Similar articles
-
Anatomical characterization of a rabbit cerebellar eyeblink premotor pathway using pseudorabies and identification of a local modulatory network in anterior interpositus.J Neurosci. 2012 Sep 5;32(36):12472-87. doi: 10.1523/JNEUROSCI.2088-12.2012. J Neurosci. 2012. PMID: 22956838 Free PMC article.
-
Distribution of premotor neurons for orbicularis oculi motoneurons in the cat, with particular reference to possible pathways for blink reflex.Neurosci Lett. 1984 Sep 7;50(1-3):251-5. doi: 10.1016/0304-3940(84)90494-4. Neurosci Lett. 1984. PMID: 6493629
-
Anatomical observations on the afferent projections to the retractor bulbi motoneuronal cell group and other pathways possibly related to the blink reflex in the cat.Brain Res. 1986 May 28;374(2):321-34. doi: 10.1016/0006-8993(86)90426-9. Brain Res. 1986. PMID: 3719341
-
Use of rabies virus as a transneuronal tracer of neuronal connections: implications for the understanding of rabies pathogenesis.Dev Biol (Basel). 2008;131:493-506. Dev Biol (Basel). 2008. PMID: 18634512 Review.
-
The cerebellum: a neuronal learning machine?Science. 1996 May 24;272(5265):1126-31. doi: 10.1126/science.272.5265.1126. Science. 1996. PMID: 8638157 Review.
Cited by
-
Caveats in Transneuronal Tracing with Unmodified Rabies Virus: An Evaluation of Aberrant Results Using a Nearly Perfect Tracing Technique.Front Neural Circuits. 2016 Jul 11;10:46. doi: 10.3389/fncir.2016.00046. eCollection 2016. Front Neural Circuits. 2016. PMID: 27462206 Free PMC article.
-
Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement.Cerebellum. 2012 Jun;11(2):457-87. doi: 10.1007/s12311-011-0331-9. Cerebellum. 2012. PMID: 22161499 Free PMC article. Review.
-
Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model.Front Cell Neurosci. 2010 Jan 4;3:19. doi: 10.3389/neuro.03.019.2009. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20126519 Free PMC article.
-
Tonic and phasic phenomena underlying eye movements during sleep in the cat.J Physiol. 2008 Jul 15;586(14):3461-77. doi: 10.1113/jphysiol.2008.153239. Epub 2008 May 22. J Physiol. 2008. PMID: 18499729 Free PMC article.
-
Trigeminal pathways for hypertonic saline- and light-evoked corneal reflexes.Neuroscience. 2014 Sep 26;277:716-23. doi: 10.1016/j.neuroscience.2014.07.052. Epub 2014 Jul 31. Neuroscience. 2014. PMID: 25086311 Free PMC article.
References
-
- Berger TW, Rinaldi P, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophysiol. 1983;50:1197–1219. - PubMed
-
- Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp Brain Res. 1986;63:341–350. - PubMed
-
- Berthier NE, Moore JW. Activity of deep cerebellar nuclear cells during classical conditioning of nictitating membrane extension in rabbits. Exp Brain Res. 1990;83:44–54. - PubMed
-
- Bour LJ, Aramideh M, De Visser BW. Neurophysiological aspects of eye and eyelid movements during blinking in humans. J Neurophysiol. 2000;83:166–176. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
