A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons
- PMID: 12388589
- PMCID: PMC6757688
- DOI: 10.1523/JNEUROSCI.22-20-08827.2002
A receptor for activated C kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs in neurons
Abstract
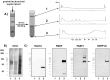
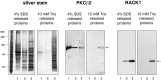
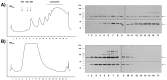
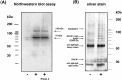
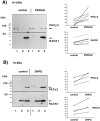
Long-lasting changes in synaptic functions after an appropriate stimulus require altered protein expression at the synapse. To restrict changes in protein composition to activated synapses, proteins may be synthesized locally as a result of transmitter receptor-triggered signaling pathways. Second messenger-controlled mechanisms that affect mRNA translation are essentially unknown. Here we report that a receptor for activated C kinase, RACK1, is a component of messenger ribonucleoprotein (mRNP) complexes. RACK1 is predominantly associated with polysome-bound, polyA-mRNAs that are being actively translated. We find it to be present in a complex with beta-tubulin and at least two mRNA-binding proteins, polyA-binding protein 1 and a 130 kDa polyA-mRNA binding protein (KIAA0217). Activation of PKCbeta2 in vitro by phosphatidylserine/diacylglycerol or in hippocampal slices by metabotropic glutamate receptor stimulation increased the amount of RACK1/PKCbeta2 associated with polysome-bound polyA-mRNAs. In vitro, PKCbeta2 can phosphorylate a subset of polyA-mRNA-associated proteins that are also phosphorylated under in vivo conditions. On the basis of these findings plus the somatodendritic localization of RACK1, we hypothesize that metabotropic glutamate receptor-triggered binding of activated PKCbeta2 to mRNP complexes bound to polyA-mRNAs is involved in activity-triggered control of protein synthesis.
Figures




References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. - PubMed
-
- Angenstein F, Hirschfelder M, Staak S. Activation of metabotropic glutamate receptors increases endogenous protein kinase C substrate phosphorylation in adult hippocampal slices. Brain Res. 1997;745:46–54. - PubMed
-
- Chang Y-WE, Traugh JA. Phosphorylation of elongation factor 1 and ribosomal protein S6 by multipotential S6 kinase and insulin stimulation of translational elongation. J Biol Chem. 1997;272:28252–28257. - PubMed
-
- Chicurel ME, Singer RH, Meyer CJ, Ingber DE. Integrin binding and mechanical tension induces movement of mRNA and ribosomes to focal adhesions. Nature. 1998;392:730–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
