Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline)
- PMID: 12388591
- PMCID: PMC6757703
- DOI: 10.1523/JNEUROSCI.22-20-08850.2002
Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline)
Abstract
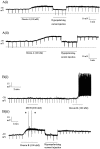
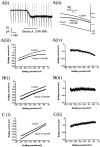
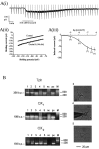
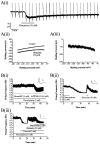
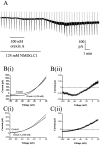
Dorsal raphe serotonin neurons fire tonically at a low rate during waking. In vitro, however, they are not spontaneously active, indicating that afferent inputs are necessary for tonic firing. Agonists of three arousal-related systems impinging on the dorsal raphe (orexin/hypocretin, histamine and the noradrenaline systems) caused an inward current and increase in current noise in whole-cell patch-clamp recordings from these neurons in brain slices. The inward current induced by all three agonists was significantly reduced in extracellular solution containing reduced sodium (25.6 mm). In extracellular recordings, all three agonists increased the firing rate of serotonin neurons; the excitatory effects of histamine and orexin A were occluded by previous application of phenylephrine, suggesting that all three systems act via common effector mechanisms. The dose-response curve for orexin B suggested an effect mediated by type II (OX2) receptors. Single-cell PCR demonstrated the presence of both OX1 and OX2 receptors in tryptophan hydroxylase-positive neurons. The effects of histamine and the adrenoceptor agonist, phenylephrine, were blocked by antagonists of histamine H1 and alpha1 receptors, respectively. The inward current induced by orexin A and phenylephrine was not blocked by protein kinase inhibitors or by thapsigargin. Three types of current-voltage responses were induced by all three agonists but in no case did the current reverse at the potassium equilibrium potential. Instead, in many cases the orexin A-induced current reversed in calcium-free medium at a value (-23 mV) consistent with the activation of a mixed cation channel (with relative permeabilities for sodium and potassium of 0.43 and 1, respectively).
Figures

References
-
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985;315:501–503. - PubMed
-
- Aghajanian GK, Lakoski JM. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984;305:181–185. - PubMed
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
