Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin
- PMID: 12388594
- PMCID: PMC6757674
- DOI: 10.1523/JNEUROSCI.22-20-08876.2002
Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin
Abstract
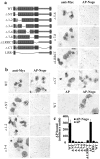
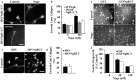
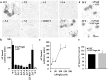
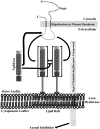
CNS myelin contains axon outgrowth inhibitors, such as Nogo, that restrict regenerative growth after injury. An understanding of the mechanism of Nogo signaling through its receptor (NgR) is critical to developing strategies for overcoming Nogo-mediated inhibition. Here we analyze the function of NgR domains in outgrowth inhibition. Analysis of alkaline phosphatase (AP)-Nogo binding in COS-7 cells reveals that the leucine-rich repeat domain is necessary and sufficient for Nogo binding and NgR multimerization. Viral infection of embryonic day 7 chick retinal ganglion cells with mutated NgR demonstrates that the NgR C-terminal domain is required for inhibitory signaling but not ligand binding. The NgR glycosylphosphatidylinositol domain is not essential for inhibitory signaling but may facilitate Nogo responses. From this analysis, we have developed a soluble, truncated version of the Nogo receptor that antagonizes outgrowth inhibition on both myelin and Nogo substrates. These data suggest that NgR mediates a significant fraction of myelin inhibition of axon outgrowth.
Figures



Similar articles
-
Nogo-66 receptor antagonist peptide promotes axonal regeneration.Nature. 2002 May 30;417(6888):547-51. doi: 10.1038/417547a. Nature. 2002. PMID: 12037567
-
Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor.Science. 2002 Aug 16;297(5584):1190-3. doi: 10.1126/science.1073031. Epub 2002 Jun 27. Science. 2002. PMID: 12089450
-
Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1.J Neurosci. 2002 Dec 1;22(23):10368-76. doi: 10.1523/JNEUROSCI.22-23-10368.2002. J Neurosci. 2002. PMID: 12451136 Free PMC article.
-
The Nogo-66 receptor: focusing myelin inhibition of axon regeneration.Trends Neurosci. 2003 Apr;26(4):193-8. doi: 10.1016/S0166-2236(03)00062-6. Trends Neurosci. 2003. PMID: 12689770 Review.
-
[Inhibitory proteins against axon regeneration in the central nervous system].Sheng Li Ke Xue Jin Zhan. 2004 Oct;35(4):311-4. Sheng Li Ke Xue Jin Zhan. 2004. PMID: 15727207 Review. Chinese.
Cited by
-
Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury.Mol Cell Neurosci. 2005 May;29(1):26-39. doi: 10.1016/j.mcn.2004.12.008. Mol Cell Neurosci. 2005. PMID: 15866044 Free PMC article.
-
New Insights into the Roles of Nogo-A in CNS Biology and Diseases.Neurochem Res. 2015 Sep;40(9):1767-85. doi: 10.1007/s11064-015-1671-5. Epub 2015 Aug 13. Neurochem Res. 2015. PMID: 26266872 Review.
-
Glial Cell-Axonal Growth Cone Interactions in Neurodevelopment and Regeneration.Front Neurosci. 2020 Mar 10;14:203. doi: 10.3389/fnins.2020.00203. eCollection 2020. Front Neurosci. 2020. PMID: 32210757 Free PMC article. Review.
-
Cortical Morphology Differences in Subjects at Increased Vulnerability for Developing a Psychotic Disorder: A Comparison between Subjects with Ultra-High Risk and 22q11.2 Deletion Syndrome.PLoS One. 2016 Nov 9;11(11):e0159928. doi: 10.1371/journal.pone.0159928. eCollection 2016. PLoS One. 2016. PMID: 27828960 Free PMC article.
-
Axonal growth inhibitors and their receptors in spinal cord injury: from biology to clinical translation.Neural Regen Res. 2023 Dec;18(12):2573-2581. doi: 10.4103/1673-5374.373674. Neural Regen Res. 2023. PMID: 37449592 Free PMC article. Review.
References
-
- Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
