Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation
- PMID: 12388598
- PMCID: PMC6757682
- DOI: 10.1523/JNEUROSCI.22-20-08911.2002
Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation
Abstract
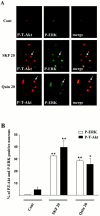
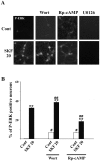
Akt is classically described as a prosurvival serine/threonine kinase activated in response to trophic factors. After activation by phosphoinositide 3-kinase (PI3-kinase), it can translocate to the nucleus where it promotes specific genetic programs by catalyzing phosphorylation of transcription factors. We report here that both dopamine (DA) D1 (SKF38393) and D2 (quinpirole) agonist treatments rapidly increase, in primary striatal neurons in culture, phosphorylation levels of Akt on Thr(308), a residue that is critically involved in its kinase activity. These treatments also activate the extracellular signal-regulated kinase (ERK) pathway in the same population of striatal neurons. Induction of active, phospho-Thr(308) Akt by dopamine D1 and D2 agonists is insensitive to wortmannin and thus PI3-kinase independent, in contrast to growth factor-induced Akt activity. D1- and D2-induced phospho-Thr(308) Akt is decreased by the mitogen-activated protein kinase kinase (MEK) inhibitor, U0126, as well as by overexpression of a dominant-negative version of MEK, thus implicating the Ras/ERK signaling cascade in this process. Furthermore, overexpression of a mutant form of Akt that cannot be activated impaired cAMP response element-binding protein (CREB) phosphorylation induced by SKF38393 and quinpirole treatments. Activation of Akt on Thr(308) was also found in vivo in striatal neurons after acute administration of cocaine, a psychostimulant that strongly increases DA transmission. Thus, multiple intracellular pathways can transduce signals from dopamine receptors to CREB in striatal neurons, one of these being Akt. We propose that this signaling pathway plays a pivotal role in DA-induced regulation of gene expression and long-term neuronal adaptation in the striatum.
Figures





References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
-
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. - PubMed
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
