Spinal neurons that possess the substance P receptor are required for the development of central sensitization
- PMID: 12388616
- PMCID: PMC6757691
- DOI: 10.1523/JNEUROSCI.22-20-09086.2002
Spinal neurons that possess the substance P receptor are required for the development of central sensitization
Abstract
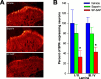
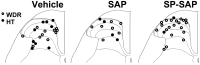
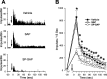
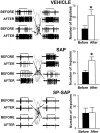
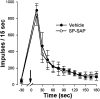
In previous studies, we have shown that loss of spinal neurons that possess the substance P receptor (SPR) attenuated pain and hyperalgesia produced by capsaicin, inflammation, and nerve injury. To determine the role of SPR-expressing neurons in modulating pain and hyperalgesia, responses of superficial and deep lumbar spinal dorsal horn neurons evoked by mechanical and heat stimuli and by capsaicin were made after ablation of SPR-expressing neurons using the selective cytotoxin conjugate substance P-saporin (SP-SAP). Morphological analysis and electrophysiological recordings were made after intrathecal infusion of vehicle, saporin alone, or SP-SAP. SP-SAP, but not vehicle or SAP alone, produced an approximately 62% decrease in SPR-expressing neurons in the dorsal horn. Loss of SPR-expressing neurons diminished the responses of remaining neurons to intraplantar injection of capsaicin. Peak responses to 10 microg of capsaicin were approximately 65% lower in animals pretreated with SP-SAP compared with controls. Additionally, sensitization to mechanical and heat stimuli that normally follows capsaicin was rarely observed. Importantly, responses to mechanical and heat stimuli in the absence of capsaicin were not altered after SP-SAP treatment. In addition, nociceptive neurons did not exhibit windup in the SP-SAP-treated group. These results demonstrate that SPR-expressing neurons located in the dorsal horn are a pivotal component of the spinal circuits involved in triggering central sensitization and hyperalgesia. It appears that this relatively small population of neurons can regulate the physiological properties of other nociceptive neurons and drive central sensitization.
Figures






Similar articles
-
Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli.Neuroscience. 2003;119(1):223-32. doi: 10.1016/s0306-4522(03)00125-8. Neuroscience. 2003. PMID: 12763083
-
Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor.Science. 1997 Oct 10;278(5336):275-9. doi: 10.1126/science.278.5336.275. Science. 1997. PMID: 9323204
-
Spinal neurons that express NK-1 receptors modulate descending controls that project through the dorsolateral funiculus.J Neurophysiol. 2005 Feb;93(2):998-1006. doi: 10.1152/jn.01160.2003. Epub 2004 Sep 29. J Neurophysiol. 2005. PMID: 15456795
-
Substance P receptor-expressing dorsal horn neurons: lessons from the targeted cytotoxin, substance P-saporin.Pain. 2008 May;136(1-2):7-10. doi: 10.1016/j.pain.2008.03.010. Epub 2008 Mar 26. Pain. 2008. PMID: 18372112 Review. No abstract available.
-
Role of neurotransmitters in sensitization of pain responses.Ann N Y Acad Sci. 2001 Mar;933:142-56. doi: 10.1111/j.1749-6632.2001.tb05821.x. Ann N Y Acad Sci. 2001. PMID: 12000017 Review.
Cited by
-
Contribution of substance P and neurokinin A to the differential injury-induced thermal and mechanical responsiveness of lamina I and V neurons.J Neurosci. 2007 Jan 24;27(4):762-70. doi: 10.1523/JNEUROSCI.2992-06.2007. J Neurosci. 2007. PMID: 17251415 Free PMC article.
-
Current and Future Issues in the Development of Spinal Agents for the Management of Pain.Curr Neuropharmacol. 2017;15(2):232-259. doi: 10.2174/1570159x14666160307145542. Curr Neuropharmacol. 2017. PMID: 26861470 Free PMC article. Review.
-
Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task.BMC Neurosci. 2017 Mar 29;18(1):36. doi: 10.1186/s12868-017-0354-3. BMC Neurosci. 2017. PMID: 28356066 Free PMC article.
-
Hyperalgesia and sensitization of dorsal horn neurons following activation of NK-1 receptors in the rostral ventromedial medulla.J Neurophysiol. 2017 Nov 1;118(5):2727-2744. doi: 10.1152/jn.00478.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794197 Free PMC article.
-
Opioids Resistance in Chronic Pain Management.Curr Neuropharmacol. 2017 Apr;15(3):444-456. doi: 10.2174/1570159X14666161101092822. Curr Neuropharmacol. 2017. PMID: 28503117 Free PMC article. Review.
References
-
- Ali Z, Wu G, Kozlov A, Barasi S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci Lett. 1996;208:203–207. - PubMed
-
- Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. - PubMed
-
- Benoliel R, Eliav E, Mannes AJ, Caudle RM, Leeman S, Iadarola MJ. Actions of intrathecal diphtheria toxin-substance P fusion protein on models of persistent pain. Pain. 1999;79:243–253. - PubMed
-
- Brown JL, Liu H, Maggio JE, Vigna SR, Mantyh PW, Basbaum AI. Morphological characterization of substance P receptor-immunoreactive neurons in the rat spinal cord and trigeminal nucleus caudalis. J Comp Neurol. 1995;356:327–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
