Roles of nonstructural protein nsP2 and Alpha/Beta interferons in determining the outcome of Sindbis virus infection
- PMID: 12388685
- PMCID: PMC136776
- DOI: 10.1128/jvi.76.22.11254-11264.2002
Roles of nonstructural protein nsP2 and Alpha/Beta interferons in determining the outcome of Sindbis virus infection
Abstract
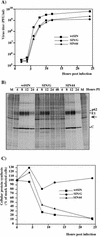
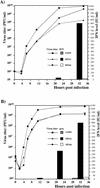
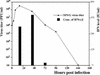
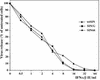
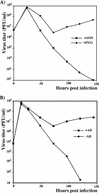
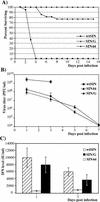
Alphaviruses productively infect a variety of vertebrate and insect cell lines. In vertebrate cells, Sindbis virus redirects cellular processes to meet the needs of virus propagation. At the same time, cells respond to virus replication by downregulating virus growth and preventing dissemination of the infection. The balance between these two mechanisms determines the outcome of infection at the cellular and organismal levels. In this report, we demonstrate that a viral nonstructural protein, nsP2, is a significant regulator of Sindbis virus-host cell interactions. This protein not only is a component of the replicative enzyme complex required for replication and transcription of viral RNAs but also plays a role in suppressing the antiviral response in Sindbis virus-infected cells. nsP2 most likely acts by decreasing interferon (IFN) production and minimizing virus visibility. Infection of murine cells with Sindbis virus expressing a mutant nsP2 leads to higher levels of IFN secretion and the activation of 170 cellular genes that are induced by IFN and/or virus replication. Secreted IFN protects naive cells against Sindbis virus infection and also stops viral replication in productively infected cells. Mutations in nsP2 can also attenuate Sindbis virus cytopathogenicity. Such mutants can persist in mammalian cells with defects in the alpha/beta IFN (IFN-alpha/beta) system or when IFN activity is neutralized by anti-IFN-alpha/beta antibodies. These findings provide new insight into the alphavirus-host cell interaction and have implications for the development of improved alphavirus expression systems with better antigen-presenting potential.
Figures


Similar articles
-
Sindbis Virus Infection Causes Cell Death by nsP2-Induced Transcriptional Shutoff or by nsP3-Dependent Translational Shutoff.J Virol. 2018 Nov 12;92(23):e01388-18. doi: 10.1128/JVI.01388-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232189 Free PMC article.
-
Decreased Virulence of Ross River Virus Harboring a Mutation in the First Cleavage Site of Nonstructural Polyprotein Is Caused by a Novel Mechanism Leading to Increased Production of Interferon-Inducing RNAs.mBio. 2018 Aug 21;9(4):e00044-18. doi: 10.1128/mBio.00044-18. mBio. 2018. PMID: 30131356 Free PMC article.
-
Persistent infection and suppression of host response by alphaviruses.Arch Virol Suppl. 2004;(18):139-47. doi: 10.1007/978-3-7091-0572-6_12. Arch Virol Suppl. 2004. PMID: 15119769 Review.
-
Comparative Characterization of the Sindbis Virus Proteome from Mammalian and Invertebrate Hosts Identifies nsP2 as a Component of the Virion and Sorting Nexin 5 as a Significant Host Factor for Alphavirus Replication.J Virol. 2018 Jun 29;92(14):e00694-18. doi: 10.1128/JVI.00694-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29743363 Free PMC article.
-
Regulators of apoptosis on the road to persistent alphavirus infection.Annu Rev Microbiol. 1997;51:565-92. doi: 10.1146/annurev.micro.51.1.565. Annu Rev Microbiol. 1997. PMID: 9343360 Review.
Cited by
-
An attenuating mutation in a neurovirulent Sindbis virus strain interacts with the IPS-1 signaling pathway in vivo.Virology. 2013 Jan 20;435(2):269-80. doi: 10.1016/j.virol.2012.09.008. Epub 2012 Oct 17. Virology. 2013. PMID: 23084425 Free PMC article.
-
Induction of GADD34 is necessary for dsRNA-dependent interferon-β production and participates in the control of Chikungunya virus infection.PLoS Pathog. 2012;8(5):e1002708. doi: 10.1371/journal.ppat.1002708. Epub 2012 May 17. PLoS Pathog. 2012. PMID: 22615568 Free PMC article.
-
The Alphaviral Capsid Protein Inhibits IRAK1-Dependent TLR Signaling.Viruses. 2021 Feb 27;13(3):377. doi: 10.3390/v13030377. Viruses. 2021. PMID: 33673546 Free PMC article.
-
Naturally Occurring and Engineered Alphaviruses Sensitive to Double-Stranded-RNA-Activated Protein Kinase Show Restricted Translation in Mammalian Cells, Increased Sensitivity to Interferon, and Marked Oncotropism.J Virol. 2020 Jan 17;94(3):e01630-19. doi: 10.1128/JVI.01630-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31723025 Free PMC article.
-
Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription.J Virol. 2004 Nov;78(22):12225-35. doi: 10.1128/JVI.78.22.12225-12235.2004. J Virol. 2004. PMID: 15507609 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

