Sialic acid functions in enterovirus 70 binding and infection
- PMID: 12388686
- PMCID: PMC136758
- DOI: 10.1128/jvi.76.22.11265-11272.2002
Sialic acid functions in enterovirus 70 binding and infection
Abstract
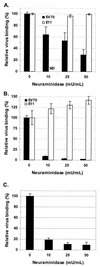
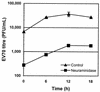
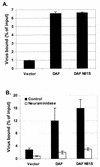
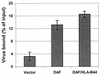
The interaction of viruses with host cell receptors is the initial step in viral infection and is an important determinant of virus host range, tissue tropism, and pathogenesis. The complement regulatory protein decay-accelerating factor (DAF/CD55) is an attachment receptor for enterovirus 70 (EV70), a member of the Picornaviridae, commonly associated with an eye infection in humans known as acute hemorrhagic conjunctivitis. In early work, the EV70 receptor on erythrocytes, responsible for its hemagglutinating activity, was shown to be sensitive to neuraminidase, implying an essential role for sialic acid in virus attachment. Here, we extend these results to show that cell surface sialic acid is required for EV70 binding to nucleated cells susceptible to virus infection and that sialic acid binding is important in productive infection. Through the use of site-directed mutagenesis to eliminate the single N-linked glycosylation site of DAF and of a chimeric receptor protein in which the O-glycosylated domain of DAF was replaced by a region of the HLA-B44 molecule, a role in EV70 binding for the sialic acid residues of DAF was excluded, suggesting the existence of at least one additional, sialylated EV70-binding factor at the cell surface. Treatment of cells with metabolic inhibitors of glycosylation excluded a role for the N-linked oligosaccharides of glycoproteins but suggested that O-linked glycosylation is important for EV70 binding.
Figures
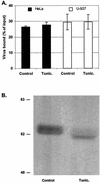
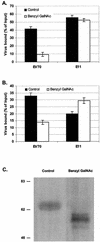
References
-
- Barton, E. S., J. L. Connolly, J. C. Forrest, J. D. Chappell, and T. S. Dermody. 2001. Utilization of sialic acid as a coreceptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem. 276:2200-2211. - PubMed
-
- Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

