Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers
- PMID: 12388703
- PMCID: PMC136789
- DOI: 10.1128/jvi.76.22.11425-11433.2002
Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers
Abstract
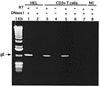
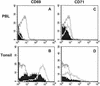
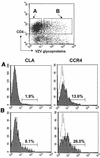
Varicella-zoster virus (VZV) is an alphaherpesvirus with the characteristic neurotropism of this group, but VZV also infects T cells productively and downregulates major histocompatibility complex (MHC) class I expression on infected T cells, as shown in the SCID-hu mouse model. T-cell tropism is likely to be critical for the cell-associated viremia associated with primary VZV infection. In these experiments, we found that VZV infects human tonsillar CD4(+) T cells in culture, with 15 to 25% being positive for VZV proteins as detected by polyclonal anti-VZV immunoglobulin G (IgG) staining and monitored by flow cytometry analysis. RNA transcripts for VZV gE, a late gene product, were detected in T-cell populations that expressed VZV surface proteins, but not in the VZV-negative cell fraction. Exposure to phorbol myristate acetate resulted in an increase in VZV-positive T cells, indicating that viral DNA was present within these cells and that VZV gene expression could be induced by T-cell activation. By immune scanning electron microscopy, VZV virions were detected in abundance on the surfaces of infected tonsillar T cells. The predominant CD4(+) T-lymphocyte subpopulations that became infected were activated CD69(+) T cells with the CD45RA(-) memory phenotype. Subsets of CD4(+) T cells that expressed skin homing markers, cutaneous leukocyte antigen, and chemokine receptor 4 were also infected with VZV. By chemotaxis assay, VZV-infected T cells migrated to SDF-1, demonstrating that skin migratory function was intact despite VZV infection. The susceptibility of tonsil T cells to VZV suggests that these cells may be important targets during the initial VZV infection of upper respiratory tract sites. Viral transfer to migrating T cells in the tonsils may facilitate cell-associated viremia, and preferential infection of CD4 T cells that express skin homing markers may enhance VZV transport to cutaneous sites of replication.
Figures





References
-
- Arvin, A. 2001. Varicella-zoster virus, p. 2731-2767. In D. Knipe and P. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
-
- Arvin, A. M., J. F. Moffat, and R. Redman. 1996. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv. Virus Res. 46:263-309. - PubMed
-
- Asano, Y., N. Itakura, Y. Hiroishi, S. Hirose, T. Nagai, T. Ozaki, T. Yazaki, K. Yamanishi, and M. Takahashi. 1985. Viremia is present in incubation period in nonimmunocompromised children with varicella. J. Pediatr. 106:69-71. - PubMed
-
- Berg, E. L., T. Yoshino, L. S. Rott, M. K. Robinson, R. A. Warnock, T. K. Kishimoto, L. J. Picker, and E. C. Butcher. 1991. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174:1461-1466. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

