Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2
- PMID: 12388712
- PMCID: PMC136768
- DOI: 10.1128/jvi.76.22.11505-11517.2002
Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2
Abstract
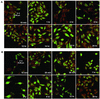
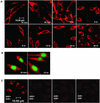
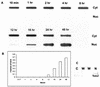
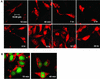
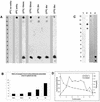
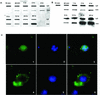
We examined cytoplasmic trafficking and nuclear translocation of adeno-associated virus type 2 (AAV) by using Alexa Fluor 488-conjugated wild-type AAV, A20 monoclonal antibody immunocytochemistry, and subcellular fractionation techniques followed by DNA hybridization. Our results indicated that in the absence of adenovirus (Ad), AAV enters the cell rapidly and escapes from early endosomes with a t(1/2) of about 10 min postinfection. Cytoplasmically distributed AAV accumulated around the nucleus and persisted perinuclearly for 16 to 24 h. Viral uncoating occurred before or during nuclear entry beginning about 12 h postinfection, when viral protein and DNA were readily detected in the nucleus. Few, if any, intact AAV capsids were found in the nucleus. In the presence of Ad, however, cytoplasmic AAV quickly translocated into the nucleus as intact particles as early as 40 min after coinfection, and this facilitated nuclear translocation of AAV was not blocked by the nuclear pore complex inhibitor thapsigargan. The rapid nuclear translocation of intact AAV capsids in the presence of Ad suggested that one or more Ad capsid proteins might be altering trafficking. Indeed, coinfection with empty Ad capsids also resulted in the appearance of AAV DNA in nuclei within 40 min. Escape from early endosomes did not seem to be affected by Ad coinfection.
Figures


Similar articles
-
Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking.J Virol. 2005 Sep;79(18):11776-87. doi: 10.1128/JVI.79.18.11776-11787.2005. J Virol. 2005. PMID: 16140755 Free PMC article.
-
Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors.J Virol. 2000 Mar;74(6):2777-85. doi: 10.1128/jvi.74.6.2777-2785.2000. J Virol. 2000. PMID: 10684294 Free PMC article.
-
Adeno-associated virus infection of murine fibroblasts with help provided by mouse adenovirus.Virology. 2009 Jul 20;390(1):22-30. doi: 10.1016/j.virol.2009.04.020. Epub 2009 May 21. Virology. 2009. PMID: 19464040 Free PMC article.
-
Nuclear Import of Adeno-Associated Viruses Imaged by High-Speed Single-Molecule Microscopy.Viruses. 2021 Jan 22;13(2):167. doi: 10.3390/v13020167. Viruses. 2021. PMID: 33499411 Free PMC article. Review.
-
Intracellular trafficking of adenovirus: many means to many ends.Adv Drug Deliv Rev. 2007 Aug 10;59(8):810-21. doi: 10.1016/j.addr.2007.06.007. Epub 2007 Jun 28. Adv Drug Deliv Rev. 2007. PMID: 17707546 Review.
Cited by
-
Adeno-Associated Virus Vector Mobilization, Risk Versus Reality.Hum Gene Ther. 2020 Oct;31(19-20):1054-1067. doi: 10.1089/hum.2020.118. Hum Gene Ther. 2020. PMID: 32829671 Free PMC article.
-
Adeno-associated virus vector as a platform for gene therapy delivery.Nat Rev Drug Discov. 2019 May;18(5):358-378. doi: 10.1038/s41573-019-0012-9. Nat Rev Drug Discov. 2019. PMID: 30710128 Free PMC article. Review.
-
Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction.J Virol. 2015 Feb;89(3):1673-87. doi: 10.1128/JVI.02520-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410859 Free PMC article.
-
Bovine AAV transcytosis inhibition by tannic acid results in functional expression of CFTR in vitro and altered biodistribution in vivo.Gene Ther. 2012 May;19(5):576-81. doi: 10.1038/gt.2011.138. Epub 2011 Oct 20. Gene Ther. 2012. PMID: 22011646 Free PMC article.
-
Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia.J Biol Chem. 2006 Oct 6;281(40):29684-92. doi: 10.1074/jbc.M604099200. Epub 2006 Aug 9. J Biol Chem. 2006. PMID: 16899463 Free PMC article.
References
-
- Agbandje, M., T. C. Jenkins, R. McKenna, A. P. Reszka, and S. Neidle. 1992. Anthracene-9,10-diones as potential anticancer agents. Synthesis, DNA-binding, and biological studies on a series of 2,6-disubstituted derivatives. J. Med. Chem. 35:1418-1429. - PubMed
-
- Bartlett, J. S., and R. J. Samulski. 1998. Fluorescent viral vectors: a new technique for the pharmacological analysis of gene therapy. Nat. Med. 4:635-637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

