Three-dimensional model of the human platelet integrin alpha IIbbeta 3 based on electron cryomicroscopy and x-ray crystallography
- PMID: 12388784
- PMCID: PMC137836
- DOI: 10.1073/pnas.212498199
Three-dimensional model of the human platelet integrin alpha IIbbeta 3 based on electron cryomicroscopy and x-ray crystallography
Abstract
Integrins are a large family of heterodimeric transmembrane signaling proteins that affect diverse biological processes such as development, angiogenesis, wound healing, neoplastic transformation, and thrombosis. We report here the three-dimensional structure at 20-A resolution of the unliganded, low-affinity state of the human platelet integrin alpha(IIb)beta(3) derived by electron cryomicroscopy and single particle image reconstruction. The large ectodomain and small cytoplasmic domains are connected by a rod of density that we interpret as two parallel transmembrane alpha-helices. The docking of the x-ray structure of the alpha(V)beta(3) ectodomain into the electron cryomicroscopy map of alpha(IIb)beta(3) requires hinge movements at linker regions between domains in the crystal structure. Comparison of the putative high- and low-affinity conformations reveals dramatic conformational changes associated with integrin activation.
Figures


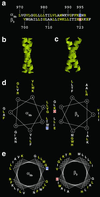
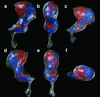
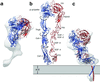
References
-
- Humphries M. J. (2000) Biochem. Soc. Trans. 28, 311-339. - PubMed
-
- Howe A., Aplin, A. E., Alahari, S. K. & Juliano, R. L. (1998) Curr. Opin. Cell Biol. 10, 220-231. - PubMed
-
- Brooks P. C., Clark, R. A. F. & Cheresh, D. A. (1994) Science 264, 569-571. - PubMed
-
- DeSimone D. W. (1994) Curr. Opin. Cell Biol. 6, 747-751. - PubMed
-
- Price D. T. & Loscalzo, J. (1999) Am. J. Med. 107, 85-97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

