The role of anti-endothelial cell antibodies in Kawasaki disease - in vitro and in vivo studies
- PMID: 12390310
- PMCID: PMC1906533
- DOI: 10.1046/j.1365-2249.2002.02000.x
The role of anti-endothelial cell antibodies in Kawasaki disease - in vitro and in vivo studies
Abstract
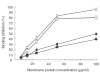
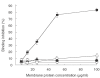
Kawasaki disease (KD) is a systemic vasculitis with cardiac and noncardiac complications. Anti--endothelial cell antibodies (AECA) are found among many patients with KD. The aim of this study was to investigate the pathogenic role of AECA in KD using in vitro and in vivo experimental models. F(ab)2 fragments of IgG-AECA and IgM-AECA were affinity purified from a patient with active KD. Their endothelial binding and ability to induce a pro-adhesive and a pro-inflammatory phenotype were evaluated in vitro. Twenty Balb/C mice were immunized with KD-AECA or with control Ig (N-Ig) to induce AECA in a murine model by the idiotypic manipulation method. Both KD-AECA isotypes bind significantly to human umbilical vein endothelial cell (HUVEC) compared to N-Ig. The in vitro activity was demonstrated by the antibodies ability to activate endothelial cells resulting in increased IL-6 secretion, adhesion molecule expression and monocytic cell line (U937) adherence to HUVEC. Five of the mice that received KD-AECA developed murine AECA after 3 months. None of the mice that received N-Ig produced AECA. The murine AECA increased monocyte adhesion to EC in vitro, similarly to the AECA used for immunization. Furthermore, all the mice that developed AECA had proteinuria and IgG deposition in the renal mesangium. No histological or immunofluorescence evidence of cardiac vasculitis could be detected. AECA might play a role in the emergence of some of KD manifestations.
Figures




Similar articles
-
IgG anti-endothelial cell autoantibodies from patients with systemic lupus erythematosus or systemic vasculitis stimulate the release of two endothelial cell-derived mediators, which enhance adhesion molecule expression and leukocyte adhesion in an autocrine manner.Arthritis Rheum. 1999 Apr;42(4):631-40. doi: 10.1002/1529-0131(199904)42:4<631::AID-ANR5>3.0.CO;2-X. Arthritis Rheum. 1999. PMID: 10211876
-
Antibodies to endothelial cells in Kawasaki disease lyse endothelial cells without cytokine pretreatment.Clin Exp Immunol. 1997 Jan;107(1):120-6. doi: 10.1046/j.1365-2249.1997.d01-894.x. Clin Exp Immunol. 1997. PMID: 9010266 Free PMC article.
-
IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines.J Clin Invest. 1996 Jan 1;97(1):111-9. doi: 10.1172/JCI118377. J Clin Invest. 1996. PMID: 8550821 Free PMC article.
-
Pathogenic role of anti-endothelial cell antibodies in systemic vasculitis.Wien Klin Wochenschr. 2000 Aug 25;112(15-16):660-4. Wien Klin Wochenschr. 2000. PMID: 11020952 Review.
-
[Detection of specific markers: our research on the marker in patients with Kawasaki disease].Nihon Rinsho Meneki Gakkai Kaishi. 2010;33(4):207-13. doi: 10.2177/jsci.33.207. Nihon Rinsho Meneki Gakkai Kaishi. 2010. PMID: 20818149 Review. Japanese.
Cited by
-
Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection.J Med Virol. 2005 Sep;77(1):1-7. doi: 10.1002/jmv.20407. J Med Virol. 2005. PMID: 16032747 Free PMC article.
-
Anti-endothelial cell antibodies in pathogenesis of vasculitis.Front Immunol. 2025 Apr 30;16:1567293. doi: 10.3389/fimmu.2025.1567293. eCollection 2025. Front Immunol. 2025. PMID: 40370444 Free PMC article. Review.
-
Kawasaki disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin.Autoimmun Rev. 2010 Apr;9(6):441-8. doi: 10.1016/j.autrev.2009.12.004. Epub 2010 Jan 13. Autoimmun Rev. 2010. PMID: 20004744 Free PMC article. Review.
-
Increased frequency of immunoglobulin (Ig)A-secreting cells following Toll-like receptor (TLR)-9 engagement in patients with Kawasaki disease.Clin Exp Immunol. 2011 Mar;163(3):346-53. doi: 10.1111/j.1365-2249.2010.04297.x. Epub 2010 Dec 22. Clin Exp Immunol. 2011. PMID: 21175593 Free PMC article.
-
Antibodies and Immunity During Kawasaki Disease.Front Cardiovasc Med. 2020 May 28;7:94. doi: 10.3389/fcvm.2020.00094. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32671098 Free PMC article. Review.
References
-
- Kawasaki T, Kosaki F, Okawa S, et al. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6. - PubMed
-
- Lacroix J, Lapointe N, Weber M, et al. Prospective study of 64 cases of Kawasaki's disease. Arch Fr Pediatr. 1985;42:771–6. - PubMed
-
- Lee BW, Tay JS, Yip WC, et al. Kawasaki syndrome in Chinese children. Ann Trop Paediatr. 1989;9:147–51. - PubMed
-
- Salcedo JR, Greenberg L, Kapur S. Renal histology of mucocutaneous lymph node syndrome (Kawasaki disease) Clin Nephrol. 1988;29:47–51. - PubMed
-
- Dhillon R, Clarkson P, Donald AE, et al. Endothelial dysfunction late after Kawasaki disease. Circulation. 1996;94:2103–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

