The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea
- PMID: 12390928
- PMCID: PMC1763069
- DOI: 10.1136/adc.87.5.438
The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea
Abstract
Aim: To review 66 children with obstructive sleep apnoea (OSA) for whom a trial of nasal continuous positive airway pressure (nCPAP) was proposed.
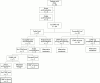
Methods: Baseline sleep studies were performed to assess OSA severity; a trial of nCPAP was performed where moderate to severe OSA, not relieved by adenotonsillectomy, was found. The nCPAP trial was considered either technically successful (ST), if the child accepted the mask for sufficient time to determine nCPAP efficacy, or a technical failure (FT) if otherwise. Patients with an initial FT were offered a period of home acclimatisation to familiarise them with wearing the mask during sleep. ST patients in whom nCPAP was effective were established on long term therapy.
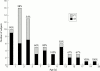
Results: Nasal CPAP trials were successful (ST) in 49/66 (74%) patients. Nasal CPAP efficacy could not be determined in the remaining 17 FT patients (26%), generally because of their poor nCPAP tolerance. These patients were subsequently considered for other treatment. A total of 42/49 (86%) ST patients were established on long term nCPAP therapy, 2/49 (4%) derived no benefit from nCPAP, while 5/49 (10%) refused long term nCPAP therapy. Of patients on long term nCPAP, the most frequently reported side effects were skin irritation and nasal dryness; however, these were not serious enough to require any patients to discontinue therapy. A period of home acclimatisation was found to be effective in increasing nCPAP acceptance, with 26% of FT children being subsequently successfully reassessed for nCPAP.
Conclusion: The use of nCPAP was feasible in a significant proportion of a paediatric OSA population. Failure was usually because of the child's intolerance of the nCPAP equipment. Nasal CPAP was an effective treatment in the majority of patients where it could be assessed, and was adopted as a long term therapy in most cases. We have successfully used nCPAP to treat OSA across a wide range of ages. Motivated parents and skilled support staff have proved essential for the success of nCPAP in a paediatric setting.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
