Nucleolin as activator of human papillomavirus type 18 oncogene transcription in cervical cancer
- PMID: 12391018
- PMCID: PMC2194043
- DOI: 10.1084/jem.20011053
Nucleolin as activator of human papillomavirus type 18 oncogene transcription in cervical cancer
Abstract
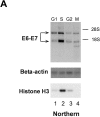
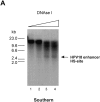
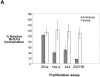
High risk human papillomaviruses (HPVs) are central to the development of cervical cancer and the deregulated expression of high risk HPV oncogenes is a critical event in this process. Here, we find that the cell protein nucleolin binds in a sequence-specific manner to the HPV18 enhancer. The DNA binding activity of nucleolin is primarily S phase specific, much like the transcription of the E6 and E7 oncoproteins of HPV18 in cervical cancer cells. Antisense inactivation of nucleolin blocks E6 and E7 oncogene transcription and selectively decreases HPV18(+) cervical cancer cell growth. Furthermore, nucleolin controls the chromatin structure of the HPV18 enhancer. In contrast, HPV16 oncogene transcription and proliferation rates of HPV16(+) SiHa cervical cancer cells are independent of nucleolin activity. Moreover, nucleolin expression is altered in HPV18(+) precancerous and cancerous tissue from the cervix uteri. Whereas nucleolin was homogeneously distributed in the nuclei of normal epithelial cells, it showed a speckled nuclear phenotype in HPV18(+) carcinomas. Thus, the host cell protein nucleolin is directly linked to HPV18-induced cervical carcinogenesis.
Figures
















Similar articles
-
Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12513-8. doi: 10.1073/pnas.97.23.12513. Proc Natl Acad Sci U S A. 2000. PMID: 11070078 Free PMC article.
-
Suppression of HPV E6 and E7 expression by BAF53 depletion in cervical cancer cells.Biochem Biophys Res Commun. 2011 Aug 26;412(2):328-33. doi: 10.1016/j.bbrc.2011.07.098. Epub 2011 Jul 29. Biochem Biophys Res Commun. 2011. PMID: 21821000
-
Suppression of tumorigenesis by transcription units expressing the antisense E6 and E7 messenger RNA (mRNA) for the transforming proteins of the human papilloma virus and the sense mRNA for the retinoblastoma gene in cervical carcinoma cells.Cancer Gene Ther. 1995 Mar;2(1):19-32. Cancer Gene Ther. 1995. PMID: 7621252
-
Cellular control of human papillomavirus oncogene transcription.Mol Carcinog. 1994 Jul;10(3):134-41. doi: 10.1002/mc.2940100304. Mol Carcinog. 1994. PMID: 8043195 Review.
-
Cellular targets of the oncoproteins encoded by the cancer associated human papillomaviruses.Princess Takamatsu Symp. 1991;22:239-48. Princess Takamatsu Symp. 1991. PMID: 1668886 Review.
Cited by
-
Nucleolin Overexpression Confers Increased Sensitivity to the Anti-Nucleolin Aptamer, AS1411.Cancer Invest. 2018;36(9-10):475-491. doi: 10.1080/07357907.2018.1527930. Epub 2018 Nov 5. Cancer Invest. 2018. PMID: 30396283 Free PMC article.
-
Acetylated NPM1 localizes in the nucleoplasm and regulates transcriptional activation of genes implicated in oral cancer manifestation.Mol Cell Biol. 2009 Sep;29(18):5115-27. doi: 10.1128/MCB.01969-08. Epub 2009 Jul 6. Mol Cell Biol. 2009. PMID: 19581289 Free PMC article.
-
Identification and characterization of nucleolin as a c-myc G-quadruplex-binding protein.J Biol Chem. 2009 Aug 28;284(35):23622-35. doi: 10.1074/jbc.M109.018028. Epub 2009 Jul 6. J Biol Chem. 2009. PMID: 19581307 Free PMC article.
-
Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin.PLoS One. 2008 Jun 18;3(6):e2518. doi: 10.1371/journal.pone.0002518. PLoS One. 2008. PMID: 18560571 Free PMC article.
-
Nucleolin regulates c-Jun/Sp1-dependent transcriptional activation of cPLA2alpha in phorbol ester-treated non-small cell lung cancer A549 cells.Nucleic Acids Res. 2008 Jan;36(1):217-27. doi: 10.1093/nar/gkm1027. Epub 2007 Nov 19. Nucleic Acids Res. 2008. PMID: 18025046 Free PMC article.
References
-
- zur Hausen, H., and E.-M. de Villiers. 1994. Human papillomaviruses. Annu. Rev. Microbiol. 48:427–447. - PubMed
-
- Shah, K.V. 1997. Human papillomaviruses and anogenital cancers. N. Engl. J. Med. 337:1386–1388. - PubMed
-
- Walboomers, J.M., M.V. Jacobs, M.M. Manos, F.X. Bosch, J.A. Kummler, K.V. Shah, P.J. Snijders, J. Peto, C.J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12–19. - PubMed
-
- Bosch, F.X., M.M. Manos, N. Munoz, M. Sherman, A.M. Jansen, J. Peto, M.H. Shiffman, V. Moreno, R. Kurman, and K.V. Shah. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) study group. J. Natl. Cancer Inst. 88:1361–1365. - PubMed
-
- Barnes, W., G. Delgado, R.J. Kurman, E.S. Petrilli, D.M. Smith, S. Ahmed, A.T. Lorincz, G.F. Temple, A.B. Jenson, and W.D. Lancaster. 1988. Possible prognostic signficance of human papillomavirus type in cervical cancer. Gynecol. Oncol. 29:267–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

