Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node
- PMID: 12391019
- PMCID: PMC2194046
- DOI: 10.1084/jem.20020991
Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node
Abstract
The major histocompatibility complex (MHC)-dependent presentation of processed tissue-specific self-antigens can contribute to either peripheral (extrathymic) tolerance or the differentiation of autoreactive T cells. Here, we have studied the MHC class II molecule presentation of gastric parietal cell (PC)-specific H(+)/K(+)-ATPase, which induces a destructive autoimmune gastritis in BALB/c mice lacking CD4(+) CD25(+) regulatory T cells. Immunofluorescence microscopy showed physical association of CD11c(+) dendritic cells (DCs) with PCs in the gastric mucosa. H(+)/K(+)-ATPase protein was found within vesicular compartments of a few CD11c(+) DCs only in the draining gastric lymph node (LN) and these antigen-containing DCs increased markedly in number with the onset of tissue destruction in autoimmune animals. Both CD8alpha(hi) and CD8alpha(lo) gastric DCs, but not peripheral or mesenteric DCs, showed evidence of constitutive in vivo processing and presentation of H(+)/K(+)-ATPase. These data provide direct support for a widely held model of local tissue antigen uptake and trafficking by DCs in normal animals and demonstrate that DCs in the draining LN can present a tissue-specific self-antigen under noninflammatory conditions without fully deleting autoreactive T cells or inducing active autoimmunity.
Figures


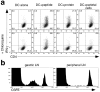



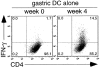
Comment in
-
Antigen presentation by dendritic cells in vivo.J Exp Med. 2002 Oct 21;196(8):1013-6. doi: 10.1084/jem.20021636. J Exp Med. 2002. PMID: 12391012 Free PMC article. No abstract available.
References
-
- von Boehmer, H. 1988. The developmental biology of T lymphocytes. Annu. Rev. Immunol. 6:309–326. - PubMed
-
- Robey, E., and B.J. Fowlkes. 1994. Selective events in T cell development. Annu. Rev. Immunol. 12:675–705. - PubMed
-
- Schwartz, R.H. 1989. Acquisition of immunologic self-tolerance. Cell. 57:1073–1081. - PubMed
-
- Schonrich, G., F. Momburg, M. Malissen, A.M. Schmitt-Verhulst, B. Malissen, G.J. Hämmerling, and B. Arnold. 1992. Distinct mechanisms of extrathymic T-cell tolerance due to differential expression of self antigen. Int. Immunol. 4:581–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

