The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens
- PMID: 12391021
- PMCID: PMC2194045
- DOI: 10.1084/jem.20020861
The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens
Abstract
We previously described a mechanism for the maintenance of peripheral self-tolerance. This involves the cross-presentation of tissue-associated antigens by a bone marrow-derived cell type that stimulates the proliferation and ultimate deletion of self-reactive CD8 T cells. This process has been referred to as cross-tolerance. Here, we characterize the elusive cell type responsible for inducing cross-tolerance as a CD8alpha(+) dendritic cell (DC). To achieve this aim, transgenic mice were generated expressing yellow fluorescent protein (YFP) linked to CTL epitopes for ovalbumin and glycoprotein B (gB) of herpes simplex virus under the rat insulin promoter (RIP). Although tracking of YFP was inconclusive, the use of a highly sensitive gB-specific hybridoma that produced beta-galactosidase on encounter with antigen, enabled detection of antigen presentation by cells isolated from the pancreatic lymph node. This showed that a CD11c(+)CD8alpha(+) cell was responsible for cross-tolerance, the same DC subset as previously implicated in cross-priming. These data indicate that CD8alpha(+) DCs play a critical role in both tolerance and immunity to cell-associated antigens, providing a potential mechanism by which cytotoxic T lymphocyte can be immunized to viral antigens while maintaining tolerance to self.
Figures




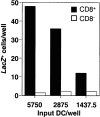
Comment in
-
Antigen presentation by dendritic cells in vivo.J Exp Med. 2002 Oct 21;196(8):1013-6. doi: 10.1084/jem.20021636. J Exp Med. 2002. PMID: 12391012 Free PMC article. No abstract available.
References
-
- Goldrath, A.W., and M.J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature. 402:255–262. - PubMed
-
- Miller, J.F., C. Kurts, J. Allison, H. Kosaka, F. Carbone, and W.R. Heath. 1998. Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens. Immunol. Rev. 165:267–277. - PubMed
-
- Matzinger, P. 1994. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12:991–1045. - PubMed
-
- Heath, W.R., and F.R. Carbone. 2001. Cross-presentation in viral immunity and self-tolerance. Nat. Rev. Immunol. 1:126–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

