Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium
- PMID: 12391028
- PMCID: PMC2173052
- DOI: 10.1083/jcb.200206096
Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium
Abstract
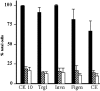
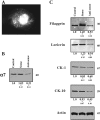
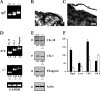
Several ganglionic nicotinic acetylcholine receptor (nAChR) types are abundantly expressed in nonneuronal locations, but their functions remain unknown. We found that keratinocyte alpha7 nAChR controls homeostasis and terminal differentiation of epidermal keratinocytes required for formation of the skin barrier. The effects of functional inactivation of alpha7 nAChR on keratinocyte cell cycle progression, differentiation, and apoptosis were studied in cell monolayers treated with alpha-bungarotoxin or antisense oligonucleotides and in the skin of Acra7 homozygous mice lacking alpha7 nAChR channels. Elimination of the alpha7 signaling pathway blocked nicotine-induced influx of 45Ca2+ and also inhibited terminal differentiation of these cells at the transcriptional and/or translational level. On the other hand, inhibition of the alpha7 nAChR pathway favored cell cycle progression. In the epidermis of alpha7-/- mice, the abnormalities in keratinocyte gene expression were associated with phenotypic changes characteristic of delayed epidermal turnover. The lack of alpha7 was associated with up-regulated expression of the alpha3 containing nAChR channels that lack alpha5 subunit, and both homomeric alpha9- and heteromeric alpha9alpha10-made nAChRs. Thus, this study demonstrates that ACh signaling through alpha7 nAChR channels controls late stages of keratinocyte development in the epidermis by regulating expression of the cell cycle progression, apoptosis, and terminal differentiation genes and that these effects are mediated, at least in part, by alterations in transmembrane Ca2+ influx.
Figures



References
-
- Arredondo, J., V.T. Nguyen, A.I. Chernyavsky, D.L. Jolkovsky, K.E. Pinkerton, and S.A. Grando. 2001. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab. Invest. 81:1653–1668. - PubMed
-
- Broide, R.S., and F.M. Leslie. 1999. The α7 nicotinic acetylcholine receptor in neuronal plasticity. Mol. Neurobiol. 20:1–16. - PubMed
-
- Broide, R.S., A. Orr-Urtreger, and J.W. Patrick. 2001. Normal apoptosis levels in mice expressing one α7 nicotinic receptor null and one L250T mutant allele. Neuroreport. 12:1643–1648. - PubMed
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

