Alternative splicing controls the mechanisms of FAK autophosphorylation
- PMID: 12391143
- PMCID: PMC134714
- DOI: 10.1128/MCB.22.22.7731-7743.2002
Alternative splicing controls the mechanisms of FAK autophosphorylation
Abstract
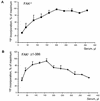
Focal adhesion kinase (FAK) is activated following integrin engagement or stimulation of transmembrane receptors. Autophosphorylation of FAK on Tyr-397 is a critical event, allowing binding of Src family kinases and activation of signal transduction pathways. Tissue-specific alternative splicing generates several isoforms of FAK with different autophosphorylation rates. Despite its importance, the mechanisms of FAK autophosphorylation and the basis for differences between isoforms are not known. We addressed these questions using isoforms of FAK expressed in brain. Autophosphorylation of FAK(+), which is identical to that of "standard" FAK, was intermolecular in transfected cells, although it did not involve the formation of stable multimeric complexes. Coumermycin-induced dimerization of gyrase B-FAK(+) chimeras triggered autophosphorylation of Tyr-397. This was independent of cell adhesion but required the C terminus of the protein. In contrast, the elevated autophosphorylation of FAK(+6,7), the major neuronal splice isoform, was not accounted for by transphosphorylation. Specifically designed immune precipitate kinase assays confirmed that autophosphorylation of FAK(+) was intermolecular, whereas autophosphorylation of FAK(+6,7) or FAK(+7) was predominantly intramolecular and insensitive to the inhibitory effects of the N-terminal domain. Our results clarify the mechanisms of FAK activation and show how alternative splicing can dramatically alter the mechanism of autophosphorylation of a protein kinase.
Figures
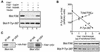

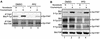





Similar articles
-
Autophosphorylation of Tyr397 and its phosphorylation by Src-family kinases are altered in focal-adhesion-kinase neuronal isoforms.Biochem J. 2000 May 15;348 Pt 1(Pt 1):119-28. Biochem J. 2000. PMID: 10794722 Free PMC article.
-
Alternatively spliced focal adhesion kinase in rat brain with increased autophosphorylation activity.J Biol Chem. 1997 Nov 7;272(45):28720-5. doi: 10.1074/jbc.272.45.28720. J Biol Chem. 1997. PMID: 9353341
-
PIAS1-mediated sumoylation of focal adhesion kinase activates its autophosphorylation.J Biol Chem. 2003 Nov 28;278(48):47434-40. doi: 10.1074/jbc.M308562200. Epub 2003 Sep 18. J Biol Chem. 2003. PMID: 14500712
-
Signaling through focal adhesion kinase.Prog Biophys Mol Biol. 1999;71(3-4):435-78. doi: 10.1016/s0079-6107(98)00052-2. Prog Biophys Mol Biol. 1999. PMID: 10354709 Review.
-
Role of focal adhesion kinase in integrin signaling.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1085-96. doi: 10.1016/s1357-2725(97)00051-4. Int J Biochem Cell Biol. 1997. PMID: 9416004 Review.
Cited by
-
Tuning cell migration: contractility as an integrator of intracellular signals from multiple cues.F1000Res. 2016 Jul 26;5:F1000 Faculty Rev-1819. doi: 10.12688/f1000research.7884.1. eCollection 2016. F1000Res. 2016. PMID: 27508074 Free PMC article. Review.
-
RhoGEFs in cell motility: novel links between Rgnef and focal adhesion kinase.Curr Mol Med. 2014 Feb;14(2):221-34. doi: 10.2174/1566524014666140128110339. Curr Mol Med. 2014. PMID: 24467206 Free PMC article. Review.
-
Significance of Cancer-Associated Fibroblasts in the Interactions of Cancer Cells with the Tumor Microenvironment of Heterogeneous Tumor Tissue.Cancers (Basel). 2023 Apr 28;15(9):2536. doi: 10.3390/cancers15092536. Cancers (Basel). 2023. PMID: 37174001 Free PMC article. Review.
-
ABI3, a component of the WAVE2 complex, is potentially regulated by PI3K/AKT pathway.Oncotarget. 2017 Jun 29;8(40):67769-67781. doi: 10.18632/oncotarget.18840. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978070 Free PMC article.
-
Reconciling the roles of FAK in osteoblast differentiation, osteoclast remodeling, and bone regeneration.Bone. 2007 Jul;41(1):39-51. doi: 10.1016/j.bone.2007.01.024. Epub 2007 Mar 13. Bone. 2007. PMID: 17459803 Free PMC article.
References
-
- Baron, V., V. Calléja, P. Ferrari, F. Alengrin, and E. Van Obberghen. 1998. p125Fak focal adhesion kinase is a substrate for the insulin and insulin-like growth factor-I tyrosine kinase receptors. J. Biol. Chem. 273:7162-7168. - PubMed
-
- Burgaya, F., and J. A. Girault. 1996. Cloning of focal adhesion kinase from rat stiatum reveals multiple transcripts. Mol. Brain Res. 37:63-73. - PubMed
-
- Burgaya, F., M. Toutant, J. M. Studler, A. Costa, M. Le Bert, M. Gelman, and J. A. Girault. 1997. Alternatively spliced focal adhesion kinase in rat brain with increased autophosphorylation activity. J. Biol. Chem. 272:28720-28725. - PubMed
-
- Calalb, M. B., X. E. Zhang, T. R. Polte, and S. K. Hanks. 1996. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem. Biophys. Res. Commun. 228:662-668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
