Direct binding of the beta1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to Ras activation
- PMID: 12391161
- PMCID: PMC134719
- DOI: 10.1128/MCB.22.22.7942-7952.2002
Direct binding of the beta1 adrenergic receptor to the cyclic AMP-dependent guanine nucleotide exchange factor CNrasGEF leads to Ras activation
Abstract
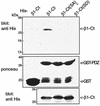
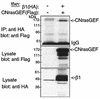
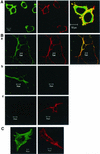
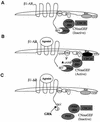
G-protein-coupled receptors (GPCRs) can indirectly activate Ras primarily through the betagamma subunits of G proteins, which recruit c-Src, phosphatidylinositol 3-kinase, and Grb2-SOS. However, a direct interaction between a Ras activator (guanine nucleotide exchange factor [GEF]) and GPCRs that leads to Ras activation has never been demonstrated. We report here a novel mechanism for a direct GPCR-mediated Ras activation. The beta1 adrenergic receptor (beta1-AR) binds to the PDZ domain of the cyclic AMP (cAMP)-dependent Ras exchange factor, CNrasGEF, via its C-terminal SkV motif. In cells heterologously expressing beta1-AR and CNrasGEF, Ras is activated by the beta1-AR agonist isoproterenol, and this activation is abolished in beta1-AR mutants that cannot bind CNrasGEF or in CNrasGEF mutants lacking the catalytic CDC25 domain or cAMP-binding domain. Moreover, the activation is transduced via Gsalpha and not via Gbetagamma. In contrast to beta1-AR, the beta2-AR neither binds CNrasGEF nor activates Ras via CNrasGEF after agonist stimulation. These results suggest a model whereby the physical interaction between the beta1-AR and CNrasGEF facilitates the transduction of Gsalpha-induced cAMP signal into the activation of Ras. The present study provides the first demonstration of direct physical association between a Ras activator and a GPCR, leading to agonist-induced Ras activation
Figures




Similar articles
-
Nedd4 regulates ubiquitination and stability of the guanine-nucleotide exchange factor CNrasGEF.J Biol Chem. 2001 Dec 14;276(50):46995-7003. doi: 10.1074/jbc.M108373200. Epub 2001 Oct 11. J Biol Chem. 2001. PMID: 11598133
-
The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP.Curr Biol. 2000 May 4;10(9):555-8. doi: 10.1016/s0960-9822(00)00473-5. Curr Biol. 2000. PMID: 10801446
-
The guanine nucleotide exchange factor CNrasGEF regulates melanogenesis and cell survival in melanoma cells.J Biol Chem. 2006 Jan 6;281(1):121-8. doi: 10.1074/jbc.M507595200. Epub 2005 Nov 4. J Biol Chem. 2006. PMID: 16272156
-
Epac- and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors.J Biol Chem. 2004 Nov 5;279(45):46497-508. doi: 10.1074/jbc.M403604200. Epub 2004 Aug 19. J Biol Chem. 2004. PMID: 15319437
-
Beta-adrenergic signaling in the heart: dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins.Sci STKE. 2001 Oct 16;2001(104):re15. doi: 10.1126/stke.2001.104.re15. Sci STKE. 2001. PMID: 11604549 Review.
Cited by
-
Protein Kinase A-independent Ras Protein Activation Cooperates with Rap1 Protein to Mediate Activation of the Extracellular Signal-regulated Kinases (ERK) by cAMP.J Biol Chem. 2016 Oct 7;291(41):21584-21595. doi: 10.1074/jbc.M116.730978. Epub 2016 Aug 16. J Biol Chem. 2016. PMID: 27531745 Free PMC article.
-
Threonine 680 phosphorylation of FLJ00018/PLEKHG2, a Rho family-specific guanine nucleotide exchange factor, by epidermal growth factor receptor signaling regulates cell morphology of Neuro-2a cells.J Biol Chem. 2014 Apr 4;289(14):10045-56. doi: 10.1074/jbc.M113.521880. Epub 2014 Feb 19. J Biol Chem. 2014. PMID: 24554703 Free PMC article.
-
Multi-functionality of proteins involved in GPCR and G protein signaling: making sense of structure-function continuum with intrinsic disorder-based proteoforms.Cell Mol Life Sci. 2019 Nov;76(22):4461-4492. doi: 10.1007/s00018-019-03276-1. Epub 2019 Aug 19. Cell Mol Life Sci. 2019. PMID: 31428838 Free PMC article. Review.
-
Insulin signaling and pharmacology in humans and in corals.PeerJ. 2024 Jan 31;12:e16804. doi: 10.7717/peerj.16804. eCollection 2024. PeerJ. 2024. PMID: 38313028 Free PMC article.
-
Separate cyclic AMP sensors for neuritogenesis, growth arrest, and survival of neuroendocrine cells.J Biol Chem. 2014 Apr 4;289(14):10126-39. doi: 10.1074/jbc.M113.529321. Epub 2014 Feb 24. J Biol Chem. 2014. PMID: 24567337 Free PMC article.
References
-
- Ambrosini, A., S. Tininini, A. Barassi, G. Racagni, E. Sturani, and R. Zippel. 2000. cAMP cascade leads to Ras activation in cortical neurons. Brain Res. Mol. Brain Res. 75:54-60. - PubMed
-
- Bogoyevitch, M. A., A. J. Ketterman, and P. H. Sugden. 1995. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J. Biol. Chem. 270:29710-29717. - PubMed
-
- Bos, J. L., J. de Rooij, and K. A. Reedquist. 2001. Rap1 signalling: adhering to new models. Nat. Rev. Mol. Cell. Biol. 2:369-377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
