Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels
- PMID: 12391325
- PMCID: PMC137905
- DOI: 10.1073/pnas.212148999
Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels
Abstract
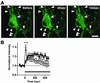
Cytoskeleton is believed to contribute to activity-dependent processes underlying neuronal plasticity, such as regulations of cellular morphology and localization of signaling proteins. However, how neuronal activity controls actin cytoskeleton remains obscure. Taking advantage of confocal imaging of enhanced GFP-actin in the primary culture of hippocampal neurons, we show that synaptic activity induces multiple types of actin reorganization, both at the spines and at the somatic periphery. Activation of N-methyl-d-aspartate receptors, accompanied with a local rise in [Ca(2+)]i, was sufficient to trigger a slow and sustained recruitment of actin into dendritic spines. In contrast, opening of voltage-gated Ca(2+) channels rapidly and reversibly enhanced cortical actin at the somatic periphery but not in the spines, in keeping with a high transient rise in somatic [Ca(2+)]i. These data suggest that spatiotemporal dynamics of [Ca(2+)]i, triggered by activation of N-methyl-d-aspartate receptors and voltage-gated Ca(2+) channels, provides the molecular basis for activity-dependent actin remodeling.
Figures

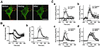


Similar articles
-
Rapid turnover of actin in dendritic spines and its regulation by activity.Nat Neurosci. 2002 Mar;5(3):239-46. doi: 10.1038/nn811. Nat Neurosci. 2002. PMID: 11850630
-
Influx of extracellular calcium regulates actin-dependent morphological plasticity in dendritic spines.Neuropharmacology. 2004 Oct;47(5):669-76. doi: 10.1016/j.neuropharm.2004.07.038. Neuropharmacology. 2004. PMID: 15458838
-
Glutamate receptors regulate actin-based plasticity in dendritic spines.Nat Neurosci. 2000 Sep;3(9):887-94. doi: 10.1038/78791. Nat Neurosci. 2000. PMID: 10966619
-
Calcium regulation of actin dynamics in dendritic spines.Cell Calcium. 2005 May;37(5):477-82. doi: 10.1016/j.ceca.2005.01.016. Cell Calcium. 2005. PMID: 15820396 Review.
-
The role of receptor/channel activity in neuronal cell migration.J Neurobiol. 1995 Mar;26(3):299-315. doi: 10.1002/neu.480260303. J Neurobiol. 1995. PMID: 7775964 Review.
Cited by
-
Prox1 inhibits neurite outgrowth during central nervous system development.Cell Mol Life Sci. 2021 Apr;78(7):3443-3465. doi: 10.1007/s00018-020-03709-2. Epub 2020 Nov 28. Cell Mol Life Sci. 2021. PMID: 33247761 Free PMC article.
-
Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade.J Neurosci. 2009 Oct 28;29(43):13720-9. doi: 10.1523/JNEUROSCI.3018-09.2009. J Neurosci. 2009. PMID: 19864584 Free PMC article.
-
Molecular determinants of acute kidney injury.J Inj Violence Res. 2015 Jul;7(2):75-86. doi: 10.5249/jivr.v7i2.615. J Inj Violence Res. 2015. PMID: 26104320 Free PMC article. Review.
-
Estradiol enhanced neuronal plasticity and ameliorated astrogliosis in human iPSC-derived neural models.Regen Ther. 2024 Jan 12;25:250-263. doi: 10.1016/j.reth.2023.12.018. eCollection 2024 Mar. Regen Ther. 2024. PMID: 38293585 Free PMC article.
-
Mechanisms of transport and exocytosis of dense-core granules containing tissue plasminogen activator in developing hippocampal neurons.J Neurosci. 2005 Mar 23;25(12):3095-106. doi: 10.1523/JNEUROSCI.4694-04.2005. J Neurosci. 2005. PMID: 15788766 Free PMC article.
References
-
- Andersen P. (1999) Nature 399, 19-21. - PubMed
-
- Smith S. J. (1999) Science 283, 1860-1861. - PubMed
-
- Blackstone C. & Sheng, M. (1999) Cell Calcium 26, 181-192. - PubMed
-
- Bliss T. V. & Collingridge, G. L. (1993) Nature 361, 31-39. - PubMed
-
- Kennedy M. B. (1998) Brain Res. Brain Res. Rev. 26, 243-257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

