Stabilities and conformations of Alzheimer's beta -amyloid peptide oligomers (Abeta 16-22, Abeta 16-35, and Abeta 10-35): Sequence effects
- PMID: 12391326
- PMCID: PMC137848
- DOI: 10.1073/pnas.212206899
Stabilities and conformations of Alzheimer's beta -amyloid peptide oligomers (Abeta 16-22, Abeta 16-35, and Abeta 10-35): Sequence effects
Abstract
Previously, we have studied the minimal oligomer size of an aggregate amyloid seed and the mechanism of seed growth with a multilayer beta-sheet model. Under high temperature simulation conditions, our approach can test the stability of possible amyloid forms. Here, we report our study of oligomers of Alzheimer's amyloid beta-peptide (Abeta) fragments 16-22, 16-35, and 10-35 (abbreviated Abeta(16-22), Abeta(16-35), and Abeta(10-35), respectively). Our simulations indicate that an antiparallel beta-sheet orientation is the most stable for the Abeta(16-22), in agreement with a solid state NMR-based model [Balbach, J. J., Ishii, Y., Antzutkin, O. N., Leapman, R. D., Rizzo, N. W., et al. (2000) Biochemistry 39, 13748-13759]. A model with twenty-four Abeta(16-22) strands indicates a highly twisted fibril. Whereas the short Abeta(16-22) and Abeta(24-36) may exist in fully extended form, the linear parallel beta-sheets for Abeta(16-35) appear impossible, mainly because of the polar region in the middle of the 16-35 sequence. However, a bent double-layered hairpin-like structure (called hook) with the polar region at the turn forms parallel beta-sheets with higher stability. An intra-strand salt-bridge (D23-K28) stabilizes the bent hairpin-like hook structure. The bent double-beta-sheet model for the Abeta(10-35) similarly offers oligomer stability.
Figures

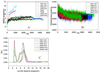


 O)… C(
O)… C( O) separations in the β-sheet oligomers of (A) octamer Antiparallel 2 model. (B) 24-mer of antiparallel sheets.
O) separations in the β-sheet oligomers of (A) octamer Antiparallel 2 model. (B) 24-mer of antiparallel sheets.



 O)… C(
O)… C( O) separations in the bent β-sheet oligomers.
O) separations in the bent β-sheet oligomers.References
-
- Carrell R. W. & Lomas, D. A. (1997) Lancet 350, 134-138. - PubMed
-
- Chesebro B. (1998) Science 279, 42-43. - PubMed
-
- Lorenzo A., Razzaboni, B., Weir, G. C. & Yankner, B. A. (1994) Nature 368, 756-760. - PubMed
-
- Tenidis K., Waldner, M., Bernhagen, J., Fischle, W., Bergmann, M., Weber, M., Merkle, M., Voelter, W., Brunner, H. & Kapurniotu, A. (1999) J. Mol. Biol. 295, 1055-1071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

